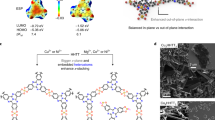
Abstract
Conductive metal–organic frameworks are an emerging class of three-dimensional architectures with degrees of modularity, synthetic flexibility and structural predictability that are unprecedented in other porous materials. However, engendering long-range charge delocalization and establishing synthetic strategies that are broadly applicable to the diverse range of structures encountered for this class of materials remain challenging. Here, we report the synthesis of K x Fe2(BDP)3 (0 ≤ x ≤ 2; BDP2− = 1,4-benzenedipyrazolate), which exhibits full charge delocalization within the parent framework and charge mobilities comparable to technologically relevant polymers and ceramics. Through a battery of spectroscopic methods, computational techniques and single-microcrystal field-effect transistor measurements, we demonstrate that fractional reduction of Fe2(BDP)3 results in a metal–organic framework that displays a nearly 10,000-fold enhancement in conductivity along a single crystallographic axis. The attainment of such properties in a K x Fe2(BDP)3 field-effect transistor represents the realization of a general synthetic strategy for the creation of new porous conductor-based devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tian, Z.-R. et al. Manganese oxide mesoporous structures: mixed-valent semiconducting catalysts. Science 276, 926–930 (1997).
Pandolfo, A. G. & Hollenkamp, A. F. Carbon properties and their role in supercapacitors. J. Power Sources 157, 11–27 (2006).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Usman, M., Mendirata, S. & Lu, K.-L. Metal–organic frameworks: new interlayer dielectric materials. ChemElectroChem 2, 786–788 (2015).
Long, J. R. & Yaghi, O. M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 38, 1213–1214 (2009).
Sculley, J., Yang, D. & Zhou, H.-C. The current status of hydrogen storage in metal–organic frameworks, updated. Energy Environ. Sci. 4, 2721–2735 (2011).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Sun, L., Campbell, M. G. & Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 55, 3566–3579 (2016).
Givaja, G., Amo-Ochoa, P., Gómez-García, C. J. & Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 41, 115–147 (2012).
Huang, X. et al. A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 6, 7480 (2015).
D’Alessandro, D. M., Kanga, J. R. R. & Caddy, J. S. Towards conducting metal–organic frameworks. Aust. J. Chem. 64, 718–722 (2011).
Darago, L. E., Aubrey, M. L., Yu, C. J., Gonzalez, M. I. & Long, J. R. Electronic conductivity, ferrimagnetic ordering, and reductive insertion mediated by organic mixed-valence in a ferric semiquinoid metal–organic framework. J. Am. Chem. Soc. 137, 15703–15711 (2015).
D’Alessandro, D. M. Exploiting redox activity in metal–organic frameworks: concepts, trends and perspectives. Chem. Commun. 52, 8957–8971 (2016).
Horike, S. et al. Fe2+-based layered porous coordination polymers and soft encapsulation of guests via redox activity. J. Mater. Chem. A 1, 3675–3679 (2013).
Kobayashi, Y., Jacobs, B., Allendorf, M. D. & Long, J. R. Conductivity, doping, and redox chemistry of a microporous dithiolene-based metal–organic framework. Chem. Mater. 22, 4120–4122 (2010).
Gándara, F. et al. Porous, conductive metal-triazolates and their structural elucidation by the charge-flipping method. Chem. Eur. J. 18, 10595–10601 (2012).
Meilikhov, M. et al. Reduction of a metal–organic framework by an organometallic complex: magnetic properties and structure of the inclusion compound [(η5-C5H5)2Co]0.5@MIL-47(V). Angew. Chem. Int. Ed. 49, 6212–6215 (2010).
Jacobson, A. J. & Nazar, L. F. in Encyclopedia of Inorganic and Bioinorganic Chemistry https://doi.org/10.1002/9781119951438.eibc0093 (Wiley, Medford, MA, 2006).
Férey, G. et al. Mixed-valence Li/Fe-based metal–organic frameworks with both reversible redox and sorption properties. Angew. Chem. Int. Ed. 46, 3259–3263 (2007).
Aubrey, M. L. & Long, J. R. A dual-ion battery cathode via oxidative insertion of anions in metal–organic framework. J. Am. Chem. Soc. 137, 13594–13602 (2015).
Kung, C.-W. et al. Metal–organic framework thin films composed of free-standing acicular nanorods exhibiting reversible electrochromism. Chem. Mater. 25, 5012–5017 (2013).
Creutz, C. Mixed valence complexes of d 5–d 6 metal centers. Prog. Inorg. Chem. 30, 1–73 (1983).
Herm, Z. R. et al. Separation of hexane isomers in a metal–organic framework with triangular channels. Science 340, 960–964 (2013).
Feller, R. K. & Cheetham, A. K. Fe(III), Mn(II), Co(II), and Ni(II) 3,4,5-trihydroxybenzoate (gallate) dihydrates; a new family of hybrid framework materials. Solid State Sci. 8, 1121–1125 (2006).
Sun, L., Hendon, C. H., Minier, M. A., Walsh, A. & Dincă, M. Million-fold electrical conductivity enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E = S, O). J. Am. Chem. Soc. 137, 6164–6167 (2015).
Mason, J. A. et al. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Torrance, J. B., Scott, B. A., Welber, B., Kauffman, F. B. & Seiden, P. E. Optical properties of the radical cation tetrathiafulvalenium (TTF+) in its mixed valence and monovalence halide salts. Phys. Rev. B 19, 730–741 (1979).
Parthey, M. & Kaupp, M. Quantum-chemical insight into mixed-valence systems within and beyond the Robin-Day scheme. Chem. Soc. Rev. 43, 5067–5088 (2014).
Collman, J. P. et al. Synthetic, electrochemical, optical, and conductivity studies of coordination polymers of iron, ruthenium, and osmium octaethylporphyrin. J. Am. Chem. Soc. 109, 4606–4614 (1987).
Tanner, D. B., Jacobsen, C. S., Garito, A. F. & Heeger, A. J. Infrared studies of the energy gap in tetrathiofulvalene-tetracyanoquinodimethane (TTF-TCNQ). Phys. Rev. B 13, 3381–3404 (1976).
Fincher, C. R. Jr., Ozaki, M., Heeger, A. J. & MacDiarmid, A. G. Donor and acceptor states in lightly doped polyacetylene, (CH) x . Phys. Rev. B 19, 4140–4148 (1979).
Masciocchi, N. et al. One-dimensional polymers containing strictly collinear metal ions: synthesis and XRPD characterization of homoleptic binary metal pyrazolates. Inorg. Chem. 41, 6080–6089 (2002).
Maxisch, T., Zhou, F. & Ceder, G. Ab initio study of the migration of small polarons in olivine Li x FePO4 and their association with lithium ions and vacancies. Phys. Rev. B 73, 104301 (2006).
Alexandrov, V., Neumann, A., Scherer, M. M. & Rosso, K. M. Electron exchange and conduction in nontronite from first-principles. J. Phys. Chem. C 117, 2032–2040 (2013).
Adelstein, N., Neaton, J. B., Asta, M. & De Jonghe, L. C. Density functional theory based calculation of small-polaron mobility in hematite. Phys. Rev. B 89, 245115-1 (2014).
Narayan, T. C., Miyaki, T., Seki, S. & Dincă, M. High charge mobility in a tetrathiafulvalene-based metal–organic framework. J. Am. Chem. Soc. 134, 12933–12935 (2012).
Saeki, A., Koizumi, Y., Aida, T. & Seki, S. Comprehensive approach to intrinsic charge carrier mobility in conjugated organic molecules, macromolecules, and supramolecular architectures. Acc. Chem. Res. 45, 1193–1202 (2012).
Saeki, A., Seki, S., Takenobu, T., Iwasa, Y. & Tagawa, S. Mobility and dynamics of charge carriers in rubrene single crystals studied by flash-photolysis microwave conductivity and optical spectroscopy. Adv. Mater. 20, 920–923 (2008).
Krebs, F. C. & Jørgensen, M. High carrier mobility in a series of new semiconducting PPV-type polymers. Macromolecules 36, 4379–4384 (2003).
Park, S. S. et al. Cation-dependent intrinsic electrical conductivity in isostructural tetrathiafulvalene-based microporous metal–organic frameworks. J. Am. Chem. Soc. 137, 1774–1777 (2015).
Talin, A. A. et al. Tunable electrical conductivity in metal–organic framework thin-film devices. Science 343, 66–69 (2014).
Sirringhaus, H. et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Kanicki, J., Libsch, F. R., Griffith, J. & Polastre, R. Performance of thin hydrogenated amorphous silicon thin-film transistors. J. Appl. Phys. 69, 2339–2345 (1991).
Bright, A., Chaikin, P. M. & McGhie, A. R. Photoconductivity and small-polaron effects in tetracyanoquinodimethane. Phys. Rev. B 10, 3560–3568 (1974).
Sheberla, D. et al. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaminotriphenylene)2, a semiconducting metal–organic graphene analogue. J. Am. Chem. Soc. 136, 8859–8862 (2014).
Wiers, B. M. Charge Transport in Metal-Organic Frameworks. PhD thesis, Univ. California Berkeley (2015).
Coelho, A. Indexing of powder diffraction patterns by iterative use of singular value decomposition. Appl. Cryst. 36, 86–95 (2003).
Cheary, R. W. & Coelho, A. A. Axial divergence in a conventional X-ray powder diffractometer. I. Theoretical foundations. J. Appl. Crystallogr. 31, 851–861 (1998).
Coelho, A. TOPAS-Academic, v.4.1 (Coelho Software 2007).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Anisimov, V. I., Aryasetiawan, F. & Lichtenstein, A. I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA + U method. J. Phys. Condens. Matter 9, 767–808 (1997).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Acknowledgements
Synthesis and characterization of the bulk materials was supported by the National Science Foundation through grant DMR-1611525. Additional efforts to synthesize the materials in nanocrystalline form were funded by a grant from the Go KRICT Project for Future Technology of the Korea Research Institute of Chemical Technology (KRICT). We thank G. Halder for assisting with powder diffraction experiments, which were collected at Beamline 17-BM-B at the Advanced Photon Source, a DoE Office of Science User Facility operated by Argonne National Laboratory under contract no. DE-AC02-06CH11357. S.E.R.-L. and S.M.H. thank R. F. Berger and K. Lee for valuable discussions. Theory and computation were supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (Theory FWP) Materials Sciences and Engineering Division (DE-AC02-05CH11231). Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. Portions of the computation work were performed at the Molecular Graphics Facility at the Department of Chemistry of UC Berkeley. Support for FP-TRMC measurements conducted by S.S. and T.S. was funded by Japan Society for the Promotion of Science (JSPS) grant no. 15K21721. The FET part was supported by the US Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division under contract no. DE-AC02-05CH11231 (PChem KC3103). We further thank Arkema for fellowship support of M.L.A., the NSF GRFP for fellowship support of L.E.D and J.A.M., and K. R. Meihaus for editing assistance.
Author information
Authors and Affiliations
Contributions
M.L.A. developed the electrochemical experiments, helped to determine the electronic structure and transport mechanism, coordinated the collaboration and wrote the manuscript. B.M.W., S.C.A., P.Y. and J.R.L. conceived of the idea and designed the study, B.M.W. synthesized and collected spectroscopic measurements, determined surface areas and coordinated the collaboration. S.C.A. fabricated and measured the FET devices, T.S. and S.S. conducted the FP-TRMC measurements, S.E.R.-L., S.M.H. and J.B.N. completed the theoretical computations, C.-J.Y conducted the electrochemical measurements, L.E.D. conducted the magnetic susceptibility measurements and helped determined the magnetic structure of the material, J.A.M. conducted the X-ray diffraction measurements, J.-O.B. helped conceive of the idea, and elucidate the transport mechanism, F.G. and G.J.L modelled the Mössbauer spectra, and determined the magnetic and electronic structure of the material. G.J.L, P.Y. and J.R.L. supervised and guided the project. All authors helped write the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Figures 1–34, Supplementary Tables 1–7, Supplementary References 1–60
Rights and permissions
About this article
Cite this article
Aubrey, M.L., Wiers, B.M., Andrews, S.C. et al. Electron delocalization and charge mobility as a function of reduction in a metal–organic framework. Nature Mater 17, 625–632 (2018). https://doi.org/10.1038/s41563-018-0098-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0098-1
This article is cited by
-
Experimental manifestation of redox-conductivity in metal-organic frameworks and its implication for semiconductor/insulator switching
Nature Communications (2023)
-
On–off conduction photoswitching in modelled spiropyran-based metal-organic frameworks
Communications Chemistry (2023)
-
Stabilizing photo-induced vacancy defects in MOF matrix for high-performance SERS detection
Nano Research (2022)
-
Current perspectives on the environmental applications using conductive metal–organic frameworks (CMOFs)
Journal of Porous Materials (2022)
-
Magnetic ordering through itinerant ferromagnetism in a metal–organic framework
Nature Chemistry (2021)