Abstract
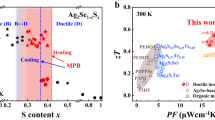
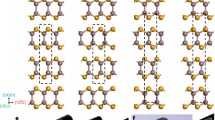
Ductility is common in metals and metal-based alloys, but is rarely observed in inorganic semiconductors and ceramic insulators. In particular, room-temperature ductile inorganic semiconductors were not known until now. Here, we report an inorganic α-Ag2S semiconductor that exhibits extraordinary metal-like ductility with high plastic deformation strains at room temperature. Analysis of the chemical bonding reveals systems of planes with relatively weak atomic interactions in the crystal structure. In combination with irregularly distributed silver–silver and sulfur–silver bonds due to the silver diffusion, they suppress the cleavage of the material, and thus result in unprecedented ductility. This work opens up the possibility of searching for ductile inorganic semiconductors/ceramics for flexible electronic devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
30 May 2018
In the version of this Article originally published, the x-axis numbers of Fig. 3d were incorrect; the range should have been 0 to 12 instead of 1 to 13. This has now been corrected.
References
Lu, L., Chen, X., Huang, X. & Lu, K. Revealing the maximum strength in nanotwinned copper. Science 323, 607–610 (2009).
Zhu, L. et al. Modeling grain size dependent optimal twin spacing for achieving ultimate high strength and related high ductility in nanotwinned metals. Acta Mater. 59, 5544–5557 (2011).
Schiøtz, J., Di Tolla, F. D. & Jacobsen, K. W. Softening of nanocrystalline metals at very small grain sizes. Nature 391, 561–563 (1998).
Taylor, G. I. The mechanism of plastic deformation of crystals. Part I. Theoretical. Proc. R. Soc. Lond. A 145, 362–387 (1934).
Green, D. J. An Introduction to the Mechanical Properties of Ceramics (Cambridge Univ. Press, Cambridge, UK, 1998).
Smithells, C. J. Smithells Metals Reference Book 7th edn (Butterworth-Heinemann, Boston, MA, USA, 1992).
Yu, P. Y. & Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties (Springer, New York, USA, 2010).
Barret, C. S. & Massalski, T. B. Structure of Metals (Pergamon, Oxford, UK, 1992).
Neamen, D. A. Semiconductor Physics and Devices: Basic Principles 3rd edn (McGraw-Hill, New York, USA, 2003).
Baca, A. J. et al. Semiconductor wires and ribbons for high-performance flexible electronics. Angew. Chem. Int. Ed. 47, 5524–5542 (2008).
Karch, J., Birringer, R. & Gleiter, H. Ceramics ductile at low temperature. Nature 330, 556–558 (1987).
Sadanaga, R. & Sueno, S. X-ray study on the α-β transition of Ag2S. Mineral. Mag. 5, 124–143 (1967).
Thaddeus, B. M. in Binary Alloy Phase Diagrams 2nd edn 2705–2708 (Materials Park, Ohio, USA, 1990).
Wang, X., Zhuang, J., Peng, Q. & Li, Y. A general strategy for nanocrystal synthesis. Nature 437, 121–124 (2005).
Junod, P., Hediger, H., Kilchör, B. & Wullschleger, J. Metal–non-metal transition in silver chalcogenides. Philos. Mag. 36, 941–958 (1977).
Lankford, J., Page, R. A. & Rabenberg, L. Deformation mechanisms in yttria-stabilized zirconia. J. Mater. Sci. 23, 4144–4156 (1988).
Barsoum, M. W. & Raghy, T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2. J. Am. Ceram. Soc. 79, 1953–1956 (1996).
Sun, Z., Zhang, Z., Hashimoto, H. & Abe, T. Ternary compound Ti3SiC2: Part II. Deformation and fracture behavior at different temperatures. Mater. Trans. 43, 432–435 (2002).
Shin, S. S., Kim, S. H. & Lee, J. C. Be-free Cu alloy exhibiting ultra-high strength and moderate tensile ductility. Mater. Sci. Eng. 639, 181–186 (2015).
Rice, J. R. & Thomson, R. Ductile versus brittle behaviour of crystals. Philos. Mag. 29, 73–97 (1974).
Bartels, T. et al. Lubricants and Lubrication. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, Weinheim, Germany, 2005).
Itakura, M. et al. Atomistic study on the cross-slip process of a screw <a> dislocation in magnesium. Model. Simul. Mater. Sci. Eng. 23, 065002 (2015).
Clouet, E., Caillard, D., Chaari, N., Onimus, F. & Rodney, D. Dislocation locking versus easy glide in titanium and zirconium. Nat. Mater. 14, 931–936 (2015).
Grin, Yu, Savin, A. & Silvi, B. in The Chemical Bond: Chemical Bonding Across the Periodic Table 18, 345–382 (Wiley, Weinheim, Germany, 2014).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Lovato, M. L. & Stout, M. G. Compression testing techniques to determine the stress/strain behavior of metals subject to finite deformation. Metall. Trans. 23, 935–951 (1992).
Schwaiger, R., Moser, B., Dao, M., Chollacoop, N. & Suresh, S. Some critical experiments on the strain-rate sensitivity of nanocrystalline nickel. Acta Mater. 51, 5159–5172 (2003).
Lu, L., Shen, Y., Chen, X., Qian, L. & Lu, K. Ultrahigh strength and high electrical conductivity in copper. Science 304, 422–426 (2004).
Kovács, I. & Vörös, G. Tensile stress–strain curves of polycrystalline silver. Phys. Status Solidi A 142, 59–67 (1994).
Shabanova, I. N. & Trapeznikov, V. A. A study of the electronic structure of Fe3C, Fe3Al and Fe3Si by x-ray photoelectron spectroscopy. J. Electron Spectrosc. 6, 297–307 (1975).
Gnanamoorthy, R., Mutoh, Y. & Mizuhara, Y. Flexural strength of gamma base titanium aluminides at room and elevated temperatures. Mater. Sci. Eng. 197, 69–77 (1995).
Akselrud, L. & Grin, Y. WinCSD: software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 47, 803–805 (2014).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133 (1965).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864 (1964).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Ceperley, D. M. & Alder, B. J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 45, 566–569 (1980).
Jepsen, O., Burkhardt, A. & Andersen, O. K. The Program TB-LMTO-ASA, 4.7 (Max-Planck-Institut für Festkörperforschung, 1999).
von Barth, U. & Hedin, L. A. Local exchange-correlation potential for the spin polarized case. J. Phys. C 5, 1629 (1972).
Andersen, O. K. Linear methods in band theory. Phys. Rev. B 12, 3060 (1975).
Lambrecht, W. R. & Andersen, O. K. Minimal basis sets in the linear muffin-tin orbital method: Application to the diamond-structure crystals C, Si, and Ge. Phys. Rev. B 34, 2439 (1986).
Kohout, M. A measure of electron localizability. Int. J. Quantum Chem. 97, 651–658 (2004).
Wagner, F. R., Bezugly, V., Kohout, M. & Grin, Y. Charge decomposition analysis of the electron localizability indicator: a bridge between the orbital and direct space representation of the chemical bond. Chem. Eur. J. 13, 5724–5741 (2007).
Kohout, M. Bonding indicators from electron pair density functionals. Faraday Discuss. 135, 43–54 (2007).
Kohout, M. Program DGrid, version 4.7 (2015).
Bader, R. F. W. Atoms in Molecules - A Quantum Theory (Clarendon, Oxford, UK, 1995).
Acknowledgements
We thank K.Lu at the Institute of Metal Research for helpful discussions. This work is supported by the National Natural Science Foundation of China (NSFC) under grant numbers 51625205 and 51632010, the Key Research Program of the Chinese Academy of Sciences (grant number KFZD-SW-421) and the Shanghai Government (grant number 15JC1400301 and 16XD1403900).
Author information
Authors and Affiliations
Contributions
F.H., R.L., T.W. and X.S. prepared the samples and measured the physical properties. H.C. performed ab initio calculations. U.B. performed microstructure experiments. Y.G. performed evaluation of X-ray diffraction measurements, structure refinements and quantum chemical calculations. X.S., H.C., R.L., Y.G. P.Q and L.C. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables: S1–S2, Supplementary Figures: Figures S1–S13
Rights and permissions
About this article
Cite this article
Shi, X., Chen, H., Hao, F. et al. Room-temperature ductile inorganic semiconductor. Nature Mater 17, 421–426 (2018). https://doi.org/10.1038/s41563-018-0047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0047-z
This article is cited by
-
Twisted-layer boron nitride ceramic with high deformability and strength
Nature (2024)
-
Origin of shear induced ‘catching bonds’ on half Heusler thermoelectric compounds XFeSb (X = Nb, Ta) and SnNiY (Y = Ti, Zr, Hf)
npj Computational Materials (2024)
-
Fully inkjet-printed Ag2Se flexible thermoelectric devices for sustainable power generation
Nature Communications (2024)
-
Breakthrough in room-temperature plastic ceramic
Science China Materials (2024)
-
SnIP-type atomic-scale inorganic double-helix semiconductors: Synthesis, properties, and applications
Nano Research (2024)