Abstract
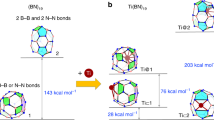
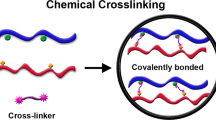
There is significant interest in the development of methods to create hybrid materials that transform capabilities, in particular for Earth-abundant metal oxides, such as TiO2, to give improved or new properties relevant to a broad spectrum of applications. Here we introduce an approach we refer to as ‘molecular cross-linking’, whereby a hybrid molecular boron oxide material is formed from polyhedral boron-cluster precursors of the type [B12(OH)12]2–. This new approach is enabled by the inherent robustness of the boron-cluster molecular building block, which is compatible with the harsh thermal and oxidizing conditions that are necessary for the synthesis of many metal oxides. In this work, using a battery of experimental techniques and materials simulation, we show how this material can be interfaced successfully with TiO2 and other metal oxides to give boron-rich hybrid materials with intriguing photophysical and electrochemical properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 March 2018
In the version of this Article originally published, Liban M. A. Saleh was incorrectly listed as Liban A. M. Saleh due to a technical error. This has now been amended in all online versions of the Article.
References
Jaffe, R. L. et al. Energy Critical Elements: Securing Materials for Emerging Technologies (Materials Research Society/American Physical Society, Washington DC, 2011).
Reddy, M. V., Subba Rao, G. V. & Chowdari, B. V. R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 113, 5364–5457 (2013).
McFarland, E. W. & Metiu, H. Catalysis by doped oxides. Chem. Rev. 113, 4391–4427 (2013).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Asahi, R., Morikawa, T., Irie, H. & Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: designs, developments, and prospects. Chem. Rev. 114, 9824–9852 (2014).
Kapilashrami, M., Zhang, Y., Liu, Y.-S., Hagfeldt, A. & Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 114, 9662–9707 (2014).
Schneider, J. et al. Understanding TiO2 photocatalysis: mechanism and materials. Chem. Rev. 114, 9919–9986 (2014).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor. Nature 238, 37–38 (1972).
Ma, Y. et al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 114, 9987–10043 (2014).
Bai, Y., Mora-Seró, I., De Angelis, F., Bisquert, J. & Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 114, 10095–10130 (2014).
Khan, S. U. M., Al-Shahry, M. & Ingler, W. B. Jr Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297, 2243–2245 (2002).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Chen, X., Liu, L. & Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 44, 1861–1885 (2015).
Salzmann, I. & Heimel, G. Toward a comprehensive understanding of molecular doping organic semiconductors. J. Electron Spectrosc. Relat. Phenom. 204, 208–222 (2015).
Pitochelli, A. R. & Hawthorne, M. F. The isolation of the icosahedral B12H12 −2 ion. J. Am. Chem. Soc. 82, 3228–3229 (1960).
Spokoyny, A. M. New ligand platforms featuring boron-rich clusters as organomimetic substituents. Pure Appl. Chem. 85, 903–919 (2013).
Sivaev, I. B., Bregadze, V. I. & Sjöberg, S. Chemistry of closo-dodecaborate anion [B12H12]2–: a review. Collect. Czech. Chem. Commun. 67, 679–727 (2002).
Hawthorne, M. F. & Pushechnikov, A. Polyhedral borane derivatives: unique and versatile structural motifs. Pure Appl. Chem. 84, 2279–2288 (2012).
Dash, B. P., Satapathy, R., Maguire, J. A. & Hosmane, N. S. Polyhedral boron clusters in materials science. New J. Chem. 35, 1955–1972 (2011).
Hansen, B. R. S., Paskevicius, M., Li, H.-W., Akiba, E. & Jensen, T. R. Metal boranes: progress and applications. Coord. Chem. Rev. 323, 60–70 (2016).
Cheng, F. & Jäkle, F. Boron-containing polymers as versatile building blocks for functional nanostructured materials. Polym. Chem. 2, 2122–2132 (2011).
Núñez, R., Romero, I., Teixidor, F. & Viñas, C. Icosahedral boron clusters: a perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 45, 5147–5173 (2016).
Alexandrova, A. N., Boldyrev, A. I., Zhai, H.-J. & Wang, L.-S. All-boron aromatic clusters as potential new inorganic and building blocks in chemistry. Coord. Chem. Rev. 250, 2811–2866 (2006).
Muetterties, E. L. Boron Hydride Chemistry (Academic, New York, NY, 1975).
Farha, O. K. et al. Synthesis of stable dodecaalkoxy derivatives of hypercloso-B12H12. J. Am. Chem. Soc. 127, 18243–18251 (2005).
Wixtrom, A. I. et al. Rapid synthesis of redox-active dodecaborane B12(OR)12 clusters under ambient conditions. Inorg. Chem. Front. 3, 711–717 (2016).
Messina, M. S. et al. Visible-light induced olefin activation using 3D aromatic boron-rich cluster photooxidants. J. Am. Chem. Soc. 138, 6952–6955 (2016).
Qian, E. A. et al. Atomically precise organomimetic cluster nanoparticles assembled via perfluoroaryl-thiol SNAr chemistry. Nat. Chem. 9, 333–340 (2017).
Pan, L. et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl Acad. Sci. USA 109, 9287–9292 (2012).
To, J. W. F. et al. Ultrahigh surface area three-dimensional porous graphitic carbon from conjugated polymeric molecular framework. ACS Cent. Sci. 1, 68–76 (2015).
Mirabelli, M. G. L. & Sneddon, L. G. Synthesis of boron carbide via poly(vinylpentaborane) precursors. J. Am. Chem. Soc. 110, 3305–3307 (1988).
Su, K. & Sneddon, L. G. A polymer precursor route to metal borides. Chem. Mater. 5, 1659–1668 (1993).
Feng, N. et al. Boron environments in B-doped and (B,N)-codoped TiO2 photocatalysts: a combined solid-state NMR and theoretical calculation study. J. Phys. Chem. C 115, 2709–2719 (2011).
Barrow, N. S. et al. Towards homonuclear J-solid-state NMR correlation experiments for half-integer quadrupolar nuclei: experimental and simulated 11B MAS spin-echo dephasing and calculated 2 J BB coupling constants for lithium diborate. Phys. Chem. Chem. Phys. 13, 5778–5789 (2011).
Billinge, S. J. L. & Kanatzidis, M. G. Beyond crystallography: the study of disorder, nanocrystallinity and crystallographically challenged materials with pair distribution functions. Chem. Commun. 0, 749–760 (2004).
Lee, M. W., Farha, O. K., Hawthorne, M. F. & Hansch, C. H. Alkoxy derivatives of dodecaborate: discrete nanomolecular ions with tunable pseudometallic properties. Angew. Chem. Int. Ed. 46, 3018–3022 (2007).
Van, N. et al. Oxidative perhydroxylation of [closo-B12H12]2– to the stable inorganic cluster redox system [B12(OH)12]2–/•–: experiment and theory. Chem. Eur. J. 16, 11242–11245 (2010).
Li, Y. et al. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotech. 7, 394–400 (2012).
Guo, Y.-G., Hu, Y.-S., Sigle, W. & Maier, J. Superior electrode performance of nanostructured mesoporous TiO2 (anatase) through efficient hierarchical mixed conducting networks. Adv. Mater. 19, 2087–2091 (2007).
Jiang, C., Hosono, E. & Zhou, H. Nanomaterials for lithium ion batteries. Nano Today 1, 28–33 November, (2006).
Gomes, A., Fernandes, E. & Lima, J. L. F. C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 65, 45–80 (2005).
Yin, Q. et al. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 (2010).
Yan, H. et al. Hybrid metal–organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly. Nat. Mater. 16, 349–355 (2017).
Bag, S., Trikalitis, P. N., Chupas, P. J., Armatas, G. S. & Kanatzidis, M. G. Porous semiconducting gels and aerogels from chalcogenide clusters. Science 317, 490–493 (2007).
Song, J. et al. A multiunit catalyst with synergistic stability and reactivity: a polyoxometalate–metal organic framework for aerobic decontamination. J. Am. Chem. Soc. 133, 16839–16846 (2011).
Yaghi, O. M., Li, G. & Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 378, 703–706 (1995).
Bachman, J. E., Smith, Z. P., Li, T., Xu, T. & Long, J. R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal-organic framework nanocrystals. Nat. Mater. 15, 845–849 (2016).
Wang, C., Xie, Z., deKrafft, K. E. & Lin, W. Doping metal–organic frameworks for water oxidation, carbon dioxide reduction and organic photocatalysis. J. Am. Chem. Soc. 133, 13445–13454 (2011).
Goellner, J. F., Gates, B. C., Vayssilov, G. N. & Rösch, N. Structure and bonding of a site-isolated transition metal complex: rhodium dicarbonyl in highly dealuminated zeolite Y. J. Am. Chem. Soc. 122, 8056–8066 (2000).
Pryor, A. Jr. et al. GENFIRE: a generalized Fourier iterative reconstruction algorithm for high-resolution 3D imaging. Sci. Rep. 7, 10409 (2017).
Yang, Y. et al. Deciphering chemical order/disorder and material properties at the single-atom level. Nature 542, 75–79 (2017).
Fung, B. M., Khitrin, A. K. & Ermolav, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 142, 97–101 (2000).
Yesinowski, J. P. Finding the true-spin-lattice relaxation time for half-integral nuclei with non-zero quadrupolar couplings. J. Mag. Reson. 252, 135–144 (2015).
Johnston, D. C. Stretched exponential relaxation arising from a continuous sum of exponential decays. Phys. Rev. B. 74, 184430 (2006).
Brouwer, D. H., Kristiansen, P. E., Fyfe, C. A. & Levitt, M. H. Symmetry-based 29Si dipolar recoupling magic angle spinning NMR spectroscopy: a new method for investigating three-dimensional structures of zeolite frameworks. J. Am. Chem. Soc. 127, 542–543 (2005).
Nicholson, R. S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 37, 1351–1355 (1965).
Moldenhauer, J., Meier, M. & Paul, D. W. Rapid and direct determination of diffusion coefficients using microelectrode arrays. J. Electrochem. Soc. 163, H672–H678 (2016).
Konopka, S. J. & McDuffie, B. Diffusion coefficients of ferri-and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 42, 1741–1746 (1970).
Acknowledgements
A.M.S. thanks the University of California, Los Angeles (UCLA), Department of Chemistry and Biochemistry for start-up funds, 3M for a Non-Tenured Faculty Award and the Alfred P. Sloan Foundation for a research fellowship in chemistry. The authors thank the MRI program of the National Science Foundation (NSF grant no. 1532232 and no.1625776) for sponsoring the acquisition of SSNMR equipment and SQUID, respectively, at UCLA. Z.J.B. was supported by a grant from the BASF Corporation, and the solid-state MAS NMR measurements at the University of California, Santa Barbara (UCSB), made use of the shared facilities of the UCSB MRSEC (NSF DMR 1720256), a member of the Materials Research Facilities Network (www.mrfn.org). E.C.W. and J.T.M. were supported by the National Science Foundation Energy Research Center for Innovative and Strategic Transformations of Alkane Resources (CISTAR) under the cooperative agreement no. EEC-1647722. J.I.Z. thanks the Student and Research Support Fund for financial support. The computational modelling benefited from access to the Extreme Science and Engineering Discovery Environment, which is supported by NSF Grant ACI-1053575. R.R.L. and M.D. were supported by the US Department of Energy (DOE), Office of Basic Energy Sciences, Division of Chemical Sciences, Biosciences and Geosciences under Contract DE-AC02-06CH11357. This research used resources of the APS, a US DOE Office of Basic Energy Sciences and Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. MRCAT operations, beamline 10-BM, are supported by the DOE and the MRCAT member institutions.
Author information
Authors and Affiliations
Contributions
A.M.S. developed the concept of molecular cross-linking and supervised the project. D.J., L.M.A.S. and A.M.S. co-designed the experiments, D.J. and L.M.A.S. performed the synthetic experimental work and D.J. performed the majority of the structural characterization and data analysis. Z.J.B. and B.F.C. designed, conducted and interpreted the SSNMR experiments and data. M.F.E.-K., J.Y.H. and, N.M. performed the electrochemical studies and interpreted the data with R.B.K. D.J., L.M.A.S., E.T., Y.S. and K.M. performed the dye degradation experiments. A.I.W. performed the EPR measurements. J.G. performed the resistivity measurements and interpreted the data with X.D. I.B.M. performed the SQUID measurements. S.K. designed and performed the STEM measurements. E.C.W. performed the XANES and EXAFS measurements and analysed the data with J.T.M. P.S.-C. and B.R. performed the mechanistic photochemical work and analysed the data with J.I.Z. R.R.L. and M.D. performed the TGA–MS and TPD ammonia experiments. J.L.B. performed the Raman spectroscopic measurements. C.H.H. performed the computational modelling. M.G.-J. and J.R. performed the TEM measurements and created the 3D reconstruction. K.W.C. collected and interpreted the high-energy X-ray scattering data . D.J., L.M.A.S., A.M.S., Z.J.B. and B.F.C. co-wrote the manuscript. All the authors discussed the results and commented on the manuscript during its preparation.
Corresponding author
Ethics declarations
Competing financial interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A correction to this article is available online at https://doi.org/10.1038/s41563-018-0054-0.
Supplementary information
Supplementary Information
Supplementary Figures 1–58, Supplementary Tables 1–4, Supplementary References 1–12
Rights and permissions
About this article
Cite this article
Jung, D., Saleh, L.M.A., Berkson, Z.J. et al. A molecular cross-linking approach for hybrid metal oxides. Nature Mater 17, 341–348 (2018). https://doi.org/10.1038/s41563-018-0021-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0021-9
This article is cited by
-
RETRACTED ARTICLE: Impact of BaFe12-0.5Cu0.5O19 on structure, elastic, morphology, composition, optical and magnetic behavior of hybrid BaFe12-0.5Cu0.5O19/Co0.6Zn0.4Fe2O4 nanocomposites
Journal of Materials Science: Materials in Electronics (2022)
-
Structural order enhances charge carrier transport in self-assembled Au-nanoclusters
Nature Communications (2020)
-
Synthesis and X-ray characterization of 15- and 16-vertex closo-carboranes
Nature Communications (2020)
-
Cross-linking dots on metal oxides
NPG Asia Materials (2019)