Abstract
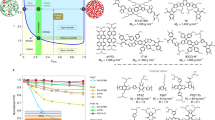
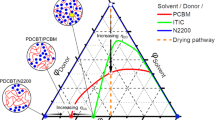
Although it is known that molecular interactions govern morphology formation and purity of mixed domains of conjugated polymer donors and small-molecule acceptors, and thus largely control the achievable performance of organic solar cells, quantifying interaction–function relations has remained elusive. Here, we first determine the temperature-dependent effective amorphous–amorphous interaction parameter, χaa(T), by mapping out the phase diagram of a model amorphous polymer:fullerene material system. We then establish a quantitative ‘constant-kink-saturation’ relation between χaa and the fill factor in organic solar cells that is verified in detail in a model system and delineated across numerous high- and low-performing materials systems, including fullerene and non-fullerene acceptors. Our experimental and computational data reveal that a high fill factor is obtained only when χaa is large enough to lead to strong phase separation. Our work outlines a basis for using various miscibility tests and future simulation methods that will significantly reduce or eliminate trial-and-error approaches to material synthesis and device fabrication of functional semiconducting blends and organic blends in general.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brédas, J.-L., Norton, J. E., Cornil, J. & Coropceanu, V. Molecular understanding of organic solar cells: the challenges. Acc. Chem. Res. 42, 1691–1699 (2009).
Huang, Y., Kramer, E. J., Heeger, A. J. & Bazan, G. C. Bulk heterojunction solar cells: morphology and performance relationships. Chem. Rev. 114, 7006–7043 (2014).
Zhao, J. et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1, 15027 (2016).
Li, S. et al. Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv. Mater. 28, 9423–9429 (2016).
Ye, L. et al. High-efficiency nonfullerene organic solar cells: critical factors that affect complex multi-length scale morphology and device performance. Adv. Energy Mater. 7, 1602000 (2017).
Baran, D. et al. Reducing the efficiency-stability-cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat. Mater. 16, 363–369 (2017).
Zhao, F. et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 29, 1700144 (2017).
Müller, C. et al. Binary organic photovoltaic blends: a simple rationale for optimum compositions. Adv. Mater. 20, 3510–3515 (2008).
Kouijzer, S. et al. Predicting morphologies of solution processed polymer:fullerene blends. J. Am. Chem. Soc. 135, 12057–12067 (2013).
Wolfer, P. et al. Identifying the optimum composition in organic solar cells comprising non-fullerene electron acceptors. J. Mater. Chem. A 1, 5989–5995 (2013).
Treat, N. D. et al. Polymer-fullerene miscibility: a metric for screening new materials for high-performance organic solar cells. J. Am. Chem. Soc. 134, 15869–15879 (2012).
Roland, S. et al. Fullerene-free polymer solar cells with highly reduced bimolecular recombination and field-independent charge carrier generation. J. Phys. Chem. Lett. 5, 2815–2822 (2014).
Ye, L. et al. Control of mesoscale morphology and photovoltaic performance in diketopyrrolopyrrole-based small band gap terpolymers. Adv. Energy Mater. 7, 1601138 (2017).
Mukherjee, S., Proctor, C. M., Bazan, G. C., Nguyen, T. Q. & Ade, H. Significance of average domain purity and mixed domains on the photovoltaic performance of high-efficiency solution-processed small-molecule BHJ solar cells. Adv. Energy Mater. 5, 1500877 (2015).
Bates, F. S. Polymer-polymer phase behavior. Science 251, 898–905 (1991).
Russell, T. P., Hjelm, R. P. & Seeger, P. A. Temperature dependence of the interaction parameter of polystyrene and poly(methyl methacrylate). Macromolecules 23, 890–893 (1990).
Leman, D. et al In situ characterization of polymer–fullerene bilayer stability. Macromolecules 48, 383–392 (2015).
Li, N. et al. Abnormal strong burn-in degradation of highly efficient polymer solar cells caused by spinodal donor-acceptor demixing. Nat. Commun. 8, 14541 (2017).
Lyons, B. P., Clarke, N. & Groves, C. The relative importance of domain size, domain purity and domain interfaces to the performance of bulk-heterojunction organic photovoltaics. Energy Environ. Sci. 5, 7657–7663 (2012).
Liu, F. et al. Molecular weight dependence of the morphology in P3HT:PCBM solar cells. ACS Appl. Mater. Interfaces 6, 19876–19887 (2014).
Bin, H. et al. 9.73% efficiency nonfullerene all organic small molecule solar cells with absorption-complementary donor and acceptor. J. Am. Chem. Soc. 139, 5085–5094 (2017).
Ghasemi, M. et al. Panchromatic sequentially cast ternary polymer solar cells. Adv. Mater. 29, 1604603 (2017).
Westacott, P. et al. Origin of fullerene-induced vitrification of fullerene:donor polymer photovoltaic blends and its impact on solar cell performance. J. Mater. Chem. A 5, 2689–2700 (2017).
Park, S. H. et al. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photon-. 3, 297–302 (2009).
Peters, C. H. et al. High efficiency polymer solar cells with long operating lifetimes. Adv. Energy Mater. 1, 491–494 (2011).
Gélinas, S. et al. Ultrafast long-range charge separation in organic semiconductor photovoltaic diodes. Science 343, 512–516 (2014).
Mukherjee, S. et al. Importance of domain purity and molecular packing in efficient solution-processed small-molecule solar cells. Adv. Mater. 27, 1105–1111 (2015).
Albrecht, S. et al. Quantifying charge extraction in organic solar cells: the case of fluorinated PCPDTBT. J. Phys. Chem. Lett. 5, 1131–1138 (2014).
Ro, H. W. et al Poly(3-hexylthiophene) and [6,6]-phenyl-C61-butyric acid methyl ester mixing in organic solar cells. Macromolecules 45, 6587–6599 (2012).
Eitouni, H. B. & Balsara, N. P. in Physical Properties of Polymers Handbook (ed. Mark, J. E.) 339–356 (Springer, New York, 2007).
Synooka, O. et al. Influence of thermal annealing on PCDTBT:PCBM composition profiles. Adv. Energy Mater. 4, 1300981 (2014).
Gann, E. et al. Soft x-ray scattering facility at the Advanced Light Source with real-time data processing and analysis. Rev. Sci. Instrum. 83, 045110 (2012).
Kozub, D. R. et al. Polymer crystallization of partially miscible polythiophene/fullerene mixtures controls morphology. Macromolecules 44, 5722–5726 (2011).
Helfand, E. & Tagami, Y. Theory of the interface between immiscible polymers. II. J. Chem. Phys. 56, 3592–3601 (1972).
Roe, R.-J. Methods of X-ray and Neutron Scattering in Polymer Science (Oxford Univ. Press, Oxford, 2000).
Nishi, T. & Wang, T. T. Melting point depression and kinetic effects of cooling on crystallization in poly(vinylidene fluoride)-poly(methyl methacrylate) mixtures. Macromolecules 8, 909–915 (1975).
Li, Z. et al. Donor polymer design enables efficient non-fullerene organic solar cells. Nat. Commun. 7, 13094 (2016).
Nielsen, C. B., Holliday, S., Chen, H.-Y., Cryer, S. J. & McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 48, 2803–2812 (2015).
Meng, D. et al. High-performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor. J. Am. Chem. Soc. 138, 375–380 (2016).
Stoltzfus, D. M., Clulow, A. J., Jin, H., Burn, P. L. & Gentle, I. R. Impact of dimerization on phase separation and crystallinity in bulk heterojunction films containing non-fullerene acceptors. Macromolecules 49, 4404–4415 (2016).
Staniec, P. A. et al. The nanoscale morphology of a PCDTBT:PCBM photovoltaic blend. Adv. Energy Mater. 1, 499–504 (2011).
Li, Z. et al. Toward improved lifetimes of organic solar cells under thermal stress: substrate-dependent morphological stability of PCDTBT:PCBM films and devices. Sci. Rep. 5, 15149 (2015).
Bartelt, J. A. et al. The importance of fullerene percolation in the mixed regions of polymer-fullerene bulk heterojunction solar cells. Adv. Energy Mater. 3, 364–374 (2013).
Ho, C. H. Y. et al. Using ultralow dosages of electron acceptor to reveal the early stage donor–acceptor electronic interactions in bulk heterojunction blends. Adv. Energy Mater. 7, 1602360 (2017).
van Franeker, J. J., Turbiez, M., Li, W., Wienk, M. M. & Janssen, R. A. A real-time study of the benefits of co-solvents in polymer solar cell processing. Nat. Commun. 6, 6229 (2015).
Dudowicz, J. & Freed, K. F. Effect of monomer structure and compressibility on the properties of multicomponent polymer blends and solutions: 1. Lattice Clust. Theory Compress. Syst. Macromol. 24, 5076–5095 (1991).
Aarts, D. G. A. L., Tuinier, R. & Lekkerkerker, H. N. W. Phase behaviour of mixtures of colloidal spheres and excluded-volume polymer chains. J. Phys. Condens. Matter 14, 7551 (2002).
Mutch, K. J., van Duijneveldt, J. S. & Eastoe, J. Colloid-polymer mixtures in the protein limit. Soft Matter 3, 155–167 (2007).
Nikolka, M. et al. High operational and environmental stability of high-mobility conjugated polymer field-effect transistors through the use of molecular additives. Nat. Mater. 16, 356–362 (2017).
Kilcoyne, A. L. D. et al. Interferometer-controlled scanning transmission X-ray microscopes at the Advanced Light Source. J. Synchrotron Radiat. 10, 125–136 (2003).
Hexemer, A. et al. A SAXS/WAXS/GISAXS beamline with multilayer monochromator. J. Phys. Conf. Ser. 247, 012007 (2010).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Jackson, N. E. et al. Conformational order in aggregates of conjugated polymers. J. Am. Chem. Soc. 137, 6254–6262 (2015).
Jorgensen, W. L. & Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1666 (1988).
Rathi, P., Huang, T.-M., Dayal, P. & Kyu, T. Crystalline−amorphous interaction in relation to the phase diagrams of binary polymer blends containing a crystalline constituent. J. Phys. Chem. B. 112, 6460–6466 (2008).
Acknowledgements
Work by NCSU was initiated with support from the US Department of Energy, Office of Science, Basic Energy Science, Division of Materials Science and Engineering under contract DE-FG02-98ER45737, and completed with support from ONR grants N00141512322 and N000141712204. X-ray data were acquired at beamlines 11.0.1.2, 7.3.3 and 5.3.2.2 at the Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract DE-AC02-05CH11231. The DSC instrument, and DSC and SIMS analysis by NCSU, were supported by a UNC General Administration Research Opportunity Initiative grant. SIMS was performed at the Analytical Instrumentation Facility (AIF) at NCSU, which is partially supported by the State of North Carolina and the National Science Foundation (award number ECCS-1542015). The AIF is a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), a site in the National Nanotechnology Coordinated Infrastructure (NNCI). The work was partially supported by the National Basic Research Program of China (973 Program; 2013CB834705), HK JEBN Limited (Hong Kong), the Hong Kong Research Grants Council (T23-407/13-N, N_HKUST623/13 and 606012), HKUST President’s Office through the SSTSP scheme (project reference number: EP201) and the National Natural Science Foundation of China (NSFC, 21374090, 21504066, 21534003 and 51320105014). The work at KAUST was supported by generous KAUST internal funding. The work at Georgia Tech was funded by ONR grant N00014-17-1-2208. A.L.D. Kilcoyne, E. Schaible, C. Zhu, A. Hexemer, C. Wang and A. Young of the ALS (DOE) assisted with the measurements and provided instrument maintenance. S. Mukherjee and O. Awartani are acknowledged for assisting with part of the X-ray data acquisition and normalization, and C. McNeill is acknowledged for initial work on the STXM miscibility measurement of PCDTBT. The KAUST IT Research Computing Team and the KAUST Supercomputing Laboratory are acknowledged for providing computational resources. The authors acknowledge and appreciate the fruitful discussions with N. Stingelin, J. Michels, E. Gomez, and M. Balik, and thank A. Dinku for maintaining the shared device fabrication facilities at NCSU, and E. Gomez for sharing his FH code.
Author information
Authors and Affiliations
Contributions
H.A. conceived the scientific framework and designed the experiments with the help of L.Y., M.G., H.H., H.Y. and J.-H.K. H.Y. initiated the study on various semi-crystalline polymer:SMA systems. H.H. made those polymer materials that were not purchased and carried out initial DSC measurements. M.G. performed DSC measurement of the PCDTBT:PCBM model system, additional DSC measurements of some semi-crystalline systems, and the SIMS measurements. L.Y. proposed the model PCDTBT system and carried out χaa–ISI–FF modelling, some DSC tests, device and R-SoXS experiments on the model system, and most of the final R-SoXS analysis. L.Y. and M.G. carried out the χaa(T) analysis. K.J. made and measured the polymer:SMA devices. J.C., J.-H.K. and T.M. acquired and analysed the initial R-SoXS data and measured materials density. B.A.C. and L.Y. acquired and analysed the STXM miscibility data. J.Y.L.L., J.Z., T.M. and Z.L. synthesized the small-molecule acceptors or building blocks. J.-L.B., T.W., X.C. and H.L. performed the MD simulations. L.Y. and H.A. wrote the manuscript. All authors contributed to editing the manuscript. H.A., J.-L.B. and H.Y. supervised the projects.
Corresponding authors
Ethics declarations
Competing financial interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supporting Information
Supplementary Information.
Rights and permissions
About this article
Cite this article
Ye, L., Hu, H., Ghasemi, M. et al. Quantitative relations between interaction parameter, miscibility and function in organic solar cells. Nature Mater 17, 253–260 (2018). https://doi.org/10.1038/s41563-017-0005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-017-0005-1
This article is cited by
-
Perovskite–organic tandem solar cells
Nature Reviews Materials (2024)
-
Lifetime over 10000 hours for organic solar cells with Ir/IrOx electron-transporting layer
Nature Communications (2023)
-
Correlating the structures and photovoltaic properties in phase-separated blends of conjugated donor polymers and acceptors
Polymer Journal (2023)
-
A materials physics perspective on structure–processing–function relations in blends of organic semiconductors
Nature Reviews Materials (2023)
-
Efficient perylene-diimides-based nonfullerene acceptors with triazine cores synthesized via a simple nucleophilic substitution reaction
Science China Materials (2023)