Abstract
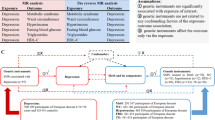
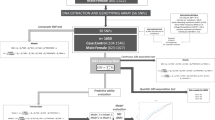
Growing evidence suggests that relative carbohydrate intake affects depression; however, the association between carbohydrates and depression remains controversial. To test this, we performed a two-sample bidirectional Mendelian randomization (MR) analysis using genetic variants associated with relative carbohydrate intake (N = 268,922) and major depressive disorder (N = 143,265) from the largest available genome-wide association studies. MR evidence suggested a causal relationship between higher relative carbohydrate intake and lower depression risk (odds ratio, 0.42 for depression per one-standard-deviation increment in relative carbohydrate intake; 95% confidence interval, 0.28 to 0.62; P = 1.49 × 10−5). Multivariable MR indicated that the protective effect of relative carbohydrate intake on depression persisted after conditioning on other diet compositions. The mediation analysis via two-step MR showed that this effect was partly mediated by body mass index, with a mediated proportion of 15.4% (95% confidence interval, 6.7% to 24.1%). These findings may inform prevention strategies and interventions directed towards relative carbohydrate intake and depression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All GWAS summary statistics analysed in this study are publicly available for download by qualified researchers. The GWASs for relative intake of carbohydrate, fat and protein can be obtained through the SSGAC data portal (https://www.thessgac.org/). The GWASs for MDD were provided by the PGC (https://www.med.unc.edu/pgc). All data generated in the current study can be obtained from the Supplementary Information.
Code availability
All the analyses used in this study were conducted using PLINK version 1.07 and R packages TwoSampleMR (version 0.4.10), MRPRESSO (version 1.0), RadialMR (version 0.4) and MendelianRandomization (version 0.5.1). The code to reproduce all results reported in the manuscript is available at https://github.com/studentyaoshi/MR.
References
Fact Sheets: Depression (World Health Organization, 2021); https://www.who.int/news-room/fact-sheets/detail/depression
Walker, E. R., McGee, R. E. & Druss, B. G. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72, 1259–1259 (2015).
Hasin, D. S. et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75, 336–346 (2018).
Hammen, C. Risk factors for depression: an autobiographical review. Annu. Rev. Clin. Psychol. 14, 1–28 (2018).
Carnegie, R. et al. Mendelian randomisation for nutritional psychiatry. Lancet Psychiatry 7, 208–216 (2020).
Huang, Q. Y., Liu, H., Suzuki, K., Ma, S. H. & Liu, C. H. Linking what we eat to our mood: a review of diet, dietary antioxidants, and depression. Antioxidants (Basel) 8, 376 (2019).
Christensen, L. The effect of food intake on mood. Clin. Nutr. 20, 161–166 (2001).
Christensen, L. & Somers, S. Comparison of nutrient intake among depressed and nondepressed individuals. Int. J. Eat. Disord. 20, 105–109 (1996).
Mantantzis, K., Schlaghecken, F., Sunram-Lea, S. I. & Maylor, E. A. Sugar rush or sugar crash? A meta-analysis of carbohydrate effects on mood. Neurosci. Biobehav. Rev. 101, 45–67 (2019).
Hu, D. Q., Cheng, L. X. & Jiang, W. J. Sugar-sweetened beverages consumption and the risk of depression: a meta-analysis of observational studies. J. Affect. Disord. 245, 348–355 (2019).
Rogers, P. J. A healthy body, a healthy mind: long-term impact of diet on mood and cognitive function. Proc. Nutr. Soc. 60, 135–143 (2001).
Pellegrin, K. L. et al. Average daily nutrient intake and mood among obese women. Nutr. Res. 18, 1103–1112 (1998).
Rosenthal, N. E. et al. Psychobiological effects of carbohydrate- and protein-rich meals in patients with seasonal affective disorder and normal controls. Biol. Psychiatry 25, 1029–1040 (1989).
Brinkworth, G. D., Buckley, J. D., Noakes, M., Clifton, P. M. & Wilson, C. J. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch. Intern. Med. 169, 1873–1880 (2009).
Daneshzad, E., Keshavarz, S. A., Qorbani, M., Larijani, B. & Azadbakht, L. Association between a low-carbohydrate diet and sleep status, depression, anxiety, and stress score. J. Sci. Food Agr. 100, 2946–2952 (2020).
Lemmens, S. G., Born, J. M., Martens, E. A., Martens, M. J. & Westerterp-Plantenga, M. S. Influence of consumption of a high-protein vs. high-carbohydrate meal on the physiological cortisol and psychological mood response in men and women. PLoS ONE 6, e16826 (2011).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Smith, G. D. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163 (2008).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Brit. Med. J. 362, k601 (2018).
Meddens, S. F. W. et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol. Psychiatry 26, 2056–2069 (2021).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
Merino, J. et al. Genetic analysis of dietary intake identifies new loci and functional links with metabolic traits. Nat. Hum. Behav. 6, 155–163 (2022).
Hartwig, F. P., Smith, G. D. & Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998 (2017).
Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 137, 119–131 (2013).
Leyton, M., Young, S. N. & Benkelfat, C. Relapse of depression after rapid depletion of tryptophan. Lancet 349, 1840–1841 (1997).
Jenkins, T. A., Nguyen, J. C. D., Polglaze, K. E. & Bertrand, P. P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut–brain axis. Nutrients 8, 56 (2016).
Fernstrom, J. D. et al. The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin. Nutr. 32, 1073–1076 (2013).
Markus, C. R. Effects of carbohydrates on brain tryptophan availability and stress performance. Biol. Psychol. 76, 83–90 (2007).
Fernstrom, J. D. & Wurtman, R. J. Brain-serotonin content—physiological regulation by plasma neutral amino-acids. Science 178, 414–416 (1972).
Rondanelli, M., Opizzi, A., Faliva, M., Bucci, M. & Perna, S. Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration. Eat. Weight Disord. 17, E22–E28 (2012).
Mikulska, J., Juszczyk, G., Gawronska-Grzywacz, M. & Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. 11, 1298 (2021).
Herrick, K. et al. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: relation to adult cortisol concentrations in the offspring. J. Clin. Endocrinol. Metab. 88, 3554–3560 (2003).
Anderson, K. E. et al. Diet–hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 40, 1761–1768 (1987).
Rodriguez, A. C. I. et al. Hypothalamic–pituitary–adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology 62, 301–318 (2015).
Pedrini, S. et al. Dietary composition modulates brain mass and solubilizable Aβ levels in a mouse model of aggressive Alzheimer’s amyloid pathology. Mol. Neurodegener. 4, 40 (2009).
Wurtman, J. & Wurtman, R. The trajectory from mood to obesity. Curr. Obes. Rep. 7, 1–5 (2018).
Freuer, D., Meisinger, C. & Linseisen, J. Causal relationship between dietary macronutrient composition and anthropometric measures: a bidirectional two-sample Mendelian randomization analysis. Clin. Nutr. 40, 4120–4131 (2021).
Hartwig, F. P. et al. Body mass index and psychiatric disorders: a Mendelian randomization study. Sci. Rep. 6, 32730 (2016).
Qi, G. H. & Chatterjee, N. Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat. Commun. 10, 1941 (2019).
Tyrrell, J. et al. Using genetics to understand the causal influence of higher BMI on depression. Int. J. Epidemiol. 48, 834–848 (2019).
van den Broek, N. et al. Causal associations between body mass index and mental health: a Mendelian randomisation study. J. Epidemiol. Community Health 72, 708–710 (2018).
Casanova, F. et al. Higher adiposity and mental health: causal inference using Mendelian randomization. Hum. Mol. Genet. 30, 2371–2382 (2021).
Speed, M. S., Jefsen, O. H., Borglum, A. D., Speed, D. & Ostergaard, S. D. Investigating the association between body fat and depression via Mendelian randomization. Transl. Psychiatry 9, 184 (2019).
Zhang, M., Chen, J., Yin, Z., Wang, L. & Peng, L. The association between depression and metabolic syndrome and its components: a bidirectional two-sample Mendelian randomization study. Transl. Psychiatry 11, 633 (2021).
Pistis, G. et al. Obesity and atypical depression symptoms: findings from Mendelian randomization in two European cohorts. Transl. Psychiatry 11, 96 (2021).
Hemani, G., Bowden, J. & Smith, G. D. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27, R195–R208 (2018).
Brumpton, B. et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat. Commun. 11, 3519 (2020).
Didelez, V. & Sheehan, N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 16, 309–330 (2007).
Angrist, J. D., Imbens, G. W. & Rubin, D. B. Identification of causal effects using instrumental variables. J. Am. Stat. Assoc. 91, 444–455 (1996).
Willett, W. Nutritional Epidemiology 3rd edn (Oxford Univ. Press, 2013).
Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40, 597–608 (2016).
Hoffmann, T. J. et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics 210, 499–515 (2018).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196 (2015).
Lu, Y. et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 7, 10495 (2016).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Staley, J. R. et al. PhenoScanner: a database of human genotype–phenotype associations. Bioinformatics 32, 3207–3209 (2016).
Kamat, M. A. et al. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics 35, 4851–4853 (2019).
Kim, H. et al. Associations between smoking, alcohol consumption, physical activity and depression in middle-aged premenopausal and postmenopausal women. Front. Psychiatry 12, 761761 (2021).
Chrzastek, Z. et al. Association of lower nutritional status and education level with the severity of depression symptoms in older adults—a cross sectional survey. Nutrients 13, 515 (2021).
Bowden, J. et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 47, 1264–1278 (2018).
Burgess, S., Thompson, S. G. & CRP CHD Genetics Collaboration Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40, 755–764 (2011).
Palmer, T. M. et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 21, 223–242 (2012).
Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43, 922–929 (2014).
Burgess, S. & Thompson, S. G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation (CRC, 2015).
Palmer, T. M. et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am. J. Epidemiol. 173, 1392–1403 (2011).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802 (2017).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Zhao, Q. Y., Wang, J. S., Hemani, G., Bowden, J. & Small, D. S. Statistical inference in two-sample summary-data mendelian randomization using robust adjusted profile score. Ann. Stat. 48, 1742–1769 (2020).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Hemani, G., Tilling, K. & Smith, G. D. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081 (2017).
Sanderson, E., Davey Smith, G., Windmeijer, F. & Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 48, 713–727 (2019).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260 (2015).
Rees, J. M. B., Wood, A. M. & Burgess, S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat. Med. 36, 4705–4718 (2017).
VanderWeele, T. J. Mediation analysis: a practitioner’s guide. Annu. Rev. Public Health 37, 17–32 (2016).
Carter, A. R. et al. Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. Brit. Med. J. 365, l1855 (2019).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos 82101601 (S.Y.), 32170616 (T.-L.Y.) and 82170896 (Y.G.)), the Natural Science Basic Research Program of Shaanxi Province (grant no. 2021JC-02 (T.-L.Y.)), Innovation Capability Support Program of Shaanxi Province (grant no. 2022TD-44 (T.-L.Y.)), China Postdoctoral Science Foundation (grant nos 2021M702612 (S.Y.), 2020M683454 (S.-S.D.) and 2021T140546 (S.-S.D.)) and the Fundamental Research Funds for the Central Universities. This study was also supported by the High-Performance Computing Platform of Xi’an Jiaotong University. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.Y. and T.-L.Y. designed the study. Y.G. and T.-L.Y. supervised the study. M.Z. participated in the data collection. S.Y., M.Z., J.-H.W., K.Z. and J.G. performed the data analyses. S.Y. and M.Z. prepared the tables and figures. S.Y. wrote the paper. S.-S.D. and Y.G. critically revised the content. All authors contributed to editing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Julien Vaucher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yao, S., Zhang, M., Dong, SS. et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav 6, 1569–1576 (2022). https://doi.org/10.1038/s41562-022-01412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-022-01412-9
This article is cited by
-
Association between modifiable lifestyle factors and telomere length: a univariable and multivariable Mendelian randomization study
Journal of Translational Medicine (2024)
-
Shared genetic architecture and causal relationship between sleep behaviors and lifespan
Translational Psychiatry (2024)
-
Causal effect of gut microbiota on the risk of prostatitis: a two-sample Mendelian randomization study
International Urology and Nephrology (2024)
-
Inferring the genetic effects of serum homocysteine and vitamin B levels on autism spectral disorder through Mendelian randomization
European Journal of Nutrition (2024)
-
The Impact of Non-alcohol Fatty Liver Disease on Bone Mineral Density is Mediated by Sclerostin by Mendelian Randomization Study
Calcified Tissue International (2024)