Abstract
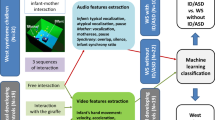
Affective speech, including motherese, captures an infant’s attention and enhances social, language and emotional development. Decreased behavioural response to affective speech and reduced caregiver–child interactions are early signs of autism in infants. To understand this, we measured neural responses to mild affect speech, moderate affect speech and motherese using natural sleep functional magnetic resonance imaging and behavioural preference for motherese using eye tracking in typically developing toddlers and those with autism. By combining diverse neural–clinical data using similarity network fusion, we discovered four distinct clusters of toddlers. The autism cluster with the weakest superior temporal responses to affective speech and very poor social and language abilities had reduced behavioural preference for motherese, while the typically developing cluster with the strongest superior temporal response to affective speech showed the opposite effect. We conclude that significantly reduced behavioural preference for motherese in autism is related to impaired development of temporal cortical systems that normally respond to parental affective speech.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The tidy data used in this study are publicly available at https://github.com/Yaqiongxiao/asdmotherese_fmriSNF.
Code availability
Completed R code for implementing all analyses reported in this article is available at https://github.com/Yaqiongxiao/asdmotherese_fmriSNF.
References
Kuhl, P. K. Is speech learning ‘gated’ by the social brain? Dev. Sci. 10, 110–120 (2007).
Kuhl, P. K. Brain mechanisms in early language acquisition. Neuron 67, 713–727 (2010).
Saint-Georges, C. et al. Motherese in interaction: at the cross-road of emotion and cognition? (A systematic review). PLoS ONE 8, 1–17 (2013).
Kuhl, P. K. et al. Cross-language analysis of phonetic units in language addressed to infants. Science 277, 684–686 (1997).
Grieser, D. A. L. & Kuhl, P. K. Maternal speech to infants in a tonal language: support for universal prosodic features in motherese. Dev. Psychol. 24, 14–20 (1988).
Falk, D. Prelinguistic evolution in early hominins: whence motherese? Behav. Brain Sci. 27, 491–541 (2004).
Cooper, R. P. & Aslin, R. N. Preference for infant-directed speech in the first month after birth. Child Dev. 61, 1584 (1990).
Fernald, A. Four-month-old infants prefer to listen to motherese. Infant Behav. Dev. 8, 181–195 (1985).
Kuhl, P. K., Coffey-Corina, S., Padden, D. & Dawson, G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev. Sci. 8, F1–F12 (2005).
Pegg, J. E., Werker, J. F. & McLeod, P. J. Preference for infant-directed over adult-directed speech: evidence from 7-week-old infants. Infant Behav. Dev. 15, 325–345 (1992).
Saito, Y. et al. Frontal cerebral blood flow change associated with infant-directed speech. Arch. Dis. Child. Fetal Neonatal Ed. 92, F113–F116 (2007).
Santesso, D. L., Schmidt, L. A. & Trainor, L. J. Frontal brain electrical activity (EEG) and heart rate in response to affective infant-directed (ID) speech in 9-month-old infants. Brain Cogn. 65, 14–21 (2007).
Sulpizio, S. et al. fNIRS reveals enhanced brain activation to female (versus male) infant directed speech (relative to adult directed speech) in young human infants. Infant Behav. Dev. 52, 89–96 (2018).
Zangl, R. & Mills, D. L. Increased brain activity to infant-directed speech in 6- and 13-month-old infants. Infancy 11, 31–62 (2007).
Zhang, Y. et al. Neural coding of formant-exaggerated speech in the infant brain. Dev. Sci. 14, 566–581 (2011).
Pierce, K. et al. Detecting, studying, and treating autism early: the one-year well-baby check-up approach. J. Pediatr. 159, 458–465.e6 (2011).
Pierce, K., Courchesne, E. & Bacon, E. To screen or not to screen universally for autism is not the question: why the task force got it wrong. J. Pediatr. 176, 182–194 (2016).
Pierce, K. et al. Evaluation of the diagnostic stability of the early autism spectrum disorder phenotype in the general population starting at 12 months. JAMA Pediatr. 173, 578–587 (2019).
Bacon, E. C. et al. Rethinking the idea of late autism spectrum disorder onset. Dev. Psychopathol. 30, 553–569 (2018).
Bruinsma, Y., Koegel, R. L. & Koegel, L. K. Joint attention and children with autism: a review of the literature. Ment. Retard. Dev. Disabil. Res. Rev. 10, 169–175 (2004).
Wang, B. et al. Similarity network fusion for aggregating data types on a genomic scale. Nat. Methods 11, 333–337 (2014).
Pai, S. & Bader, G. D. Patient similarity networks for precision medicine. J. Mol. Biol. 430, 2924–2938 (2018).
Lombardo, M. V. et al. Different functional neural substrates for good and poor language outcome in autism. Neuron 86, 267–277 (2015).
Lombardo, M. V. et al. Large-scale associations between the leukocyte transcriptome and BOLD responses to speech differ in autism early language outcome subtypes. Nat. Neurosci. 21, 1680–1688 (2018).
Klin, A. Listening preferences in regard to speech in four children with developmental disabilities. J. Child Psychol. Psychiatry 33, 763–769 (1992).
Klin, A. Young autistic children’s listening preferences in regard to speech: a possible characterization of the symptom of social withdrawal. J. Autism Dev. Disord. 21, 29–42 (1991).
Ferjan Ramírez, N., Lytle, S. R., Fish, M. & Kuhl, P. K. Parent coaching at 6 and 10 months improves language outcomes at 14 months: a randomized controlled trial. Dev. Sci. 22, e12762 (2019).
Ferjan Ramírez, N., Lytle, S. R. & Kuhl, P. K. Parent coaching increases conversational turns and advances infant language development. Proc. Natl Acad. Sci. USA 117, 3484–3491 (2020).
Bacon, E. C. et al. Measuring outcome in an early intervention program for toddlers with autism spectrum disorder: use of a curriculum-based assessment. Autism Res. Treat. 2014, 964704 (2014).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the early start Denver model. Pediatrics 125, e17–e23 (2010).
Kasari, C., Freeman, S. & Paparella, T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. J. Child Psychol. Psychiatry Allied Discip. 47, 611–620 (2006).
Sandin, S. et al. The heritability of autism spectrum disorder. J. Am. Med. Assoc. 318, 1182–1184 (2017).
Bai, D. et al. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry 76, 1035–1043 (2019).
Courchesne, E. et al. The ASD living biology: from cell proliferation to clinical phenotype. Mol. Psychiatry 24, 88–107 (2019).
Courchesne, E., Gazestani, V. H. & Lewis, N. E. Prenatal origins of ASD: the when, what, and how of ASD development. Trends Neurosci. 43, 326–342 (2020).
Gazestani, V. H. et al. A perturbed gene network containing PI3K–AKT, RAS–ERK and WNT-β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat. Neurosci. 22, 1624–1634 (2019).
Lombardo, M. V. et al. Atypical genomic patterning of the cerebral cortex in autism with poor early language outcome. Sci. Adv. 7, eabh1663 (2021).
Vernetti, A. et al. Simulating interaction: using gaze-contingent eye-tracking to measure the reward value of social signals in toddlers with and without autism. Dev. Cogn. Neurosci. 29, 21–29 (2018).
Manning, J. H., Courchesne, E. & Fox, P. T. Intrinsic connectivity network mapping in young children during natural sleep. Neuroimage 83, 288–293 (2013).
Buckley, A. W. et al. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch. Pediatr. Adolesc. Med. 164, 1032–1037 (2010).
Devnani, P. A. & Hegde, A. U. Autism and sleep disorders. J. Pediatr. Neurosci. 10, 304–307 (2015).
Goldman, S. E. et al. Defining the sleep phenotype in children with autism. Dev. Neuropsychol. 34, 560–573 (2009).
Lehoux, T., Carrier, J. & Godbout, R. NREM sleep EEG slow waves in autistic and typically developing children: morphological characteristics and scalp distribution. J. Sleep. Res. 28, 1–6 (2019).
Redcay, E. & Courchesne, E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry 64, 589–598 (2008).
Eyler, L. T., Pierce, K., Courchesne, E., Cheng, A. & Barnes, C. C. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 135, 949–960 (2012).
Pierce, K. et al. Get SET early to identify and treatment refer autism spectrum disorder at 1 year and discover factors that influence early diagnosis. J. Pediatr. 236, 179–188 (2021).
Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 392, 508–520 (2018).
Mullen, E. M. Mullen Scales of Early Learning (American Guidance Service, 1995).
Sparrow, S., Cicchetti, D. & Balla, D. Vineland-II Scales of Adaptive Behavior: Survey Form Manual (American Guidance Service, 2005).
Dehaene-Lambertz, G., Dehaene, S. & Hertz-Pannier, L. Functional neuroimaging of speech perception in infants. Science 298, 2013–2015 (2002).
Redcay, E., Kennedy, D. P. & Courchesne, E. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage 38, 696–707 (2007).
Kundu, P., Inati, S. J., Evans, J. W., Luh, W. M. & Bandettini, P. A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60, 1759–1770 (2012).
Kundu, P. et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl Acad. Sci. USA 110, 16187–16192 (2013).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Shi, F. et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS ONE 6, e18746 (2011).
Kundu, P. et al. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage 154, 59–80 (2017).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Chen, G., Adleman, N. E., Saad, Z. S., Leibenluft, E. & Cox, R. W. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage 99, 571–588 (2014).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech: Theory Exp. 2008, P10008 (2008).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
We thank the parents and children in San Diego who participated in our research, without whom this would not be possible. We are also fortunate to work with wonderful paediatricians and family practice physicians spanning a range of medical groups including UCSD, Sharp Rees-Stealy, Scripps, Rady-Children’s Primary Care Medical Group, Chula Vista Pediatrics, Graybill Medical Group, Grossmont Pediatrics, Linda Vista Health Care Center, Mills Pediatrics, North County Health Services, San Diego Family Care and Sea Breeze Pediatrics. We are grateful for their support. This work was supported by NIDCD grant 1R01DC016385 awarded to E.C. and K.P.; NIMH grants R01MH118879 and R01MH104446 awarded to K.P.; and 755816 European Research Council awarded to M.V.L. and E.C.. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.C., K.P., L.T.E. and L.K. conceived the idea and designed the study. L.K., D.G., T.H.W. and K.V. recruited the participants. L.K., D.G., T.H.W., Y.X., L.T.E. and E.C. collected the data. Y.X. conceived and performed all analyses. E.C., M.V.L. and N.E.L. aided in data analyses. E.C., K.P. and M.V.L. obtained grant funding. Y.X. and E.C. wrote the manuscript. All authors contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Human Behaviour thanks Laura Edwards and Giorgia Silani for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Tables 1–4 and Figs. 1–5.
Rights and permissions
About this article
Cite this article
Xiao, Y., Wen, T.H., Kupis, L. et al. Neural responses to affective speech, including motherese, map onto clinical and social eye tracking profiles in toddlers with ASD. Nat Hum Behav 6, 443–454 (2022). https://doi.org/10.1038/s41562-021-01237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-021-01237-y
This article is cited by
-
Infant feeding practices and autism spectrum disorder in US children aged 2–5 years: the national survey of children’s health (NSCH) 2016–2020
International Breastfeeding Journal (2023)
-
Atypical functional connectivity of temporal cortex with precuneus and visual regions may be an early-age signature of ASD
Molecular Autism (2023)
-
The Musical Turn in Biosemiotics
Biosemiotics (2023)