Abstract
Previous research points to the heritability of risk-taking behaviour. However, evidence on how genetic dispositions are translated into risky behaviour is scarce. Here, we report a genetically informed neuroimaging study of real-world risky behaviour across the domains of drinking, smoking, driving and sexual behaviour in a European sample from the UK Biobank (N = 12,675). We find negative associations between risky behaviour and grey-matter volume in distinct brain regions, including amygdala, ventral striatum, hypothalamus and dorsolateral prefrontal cortex (dlPFC). These effects are replicated in an independent sample recruited from the same population (N = 13,004). Polygenic risk scores for risky behaviour, derived from a genome-wide association study in an independent sample (N = 297,025), are inversely associated with grey-matter volume in dlPFC, putamen and hypothalamus. This relation mediates roughly 2.2% of the association between genes and behaviour. Our results highlight distinct heritable neuroanatomical features as manifestations of the genetic propensity for risk taking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data and materials are available via UKB at http://www.ukbiobank.ac.uk/.
Code availability
The analysis code used in this study is publicly available at https://osf.io/qkp4g/.
References
Knight, F. H. Risk, Uncertainty, and Profit (Houghton Mifflin, 1921).
Eckel, C. C. & Füllbrunn, S. C. Thar SHE blows? gender, competition, and bubbles in experimental asset markets. Am. Economic Rev. 105, 906–920 (2015).
Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States (Centers for Disease Control and Prevention, 2013); https://www.cdc.gov/std/stats/sti-estimates-fact-sheet-feb-2013.pdf
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E. & Brewer, R. D. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 49, e73–e79 (2015).
Blincoe, L., Miller, T. R., Zaloshnja, E. & Lawrence, B. A. The Economic and Societal Impact of Motor Vehicle Crashes, 2010 (revised) (Transportation Research Board, 2015); https://trid.trb.org/view/1311862
The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA (US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014).
Karlsson Linnér, R. et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat. Genet. 51, 245–257 (2019).
Elliott, L. T. et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216 (2018).
Thompson, P. M. et al. Genetic influences on brain structure. Nat. Neurosci. 4, 1253–1258 (2001).
Grubb, M. A., Tymula, A., Gilaie-Dotan, S., Glimcher, P. W. & Levy, I. Neuroanatomy accounts for age-related changes in risk preferences. Nat. Commun. 7, 13822 (2016).
Jung, W. H., Lee, S., Lerman, C. & Kable, J. W. Amygdala functional and structural connectivity predicts individual risk tolerance. Neuron 98, 394–404.e4 (2018).
Nasiriavanaki, Z. et al. Prediction of individual differences in risky behavior in young adults via variations in local brain structure. Front. Neurosci. 9, 359 (2015).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Marek, S. et al. Towards reproducible brain-wide association studies. Preprint at bioRxiv https://doi.org/10.1101/2020.08.21.257758 (2020).
Dohmen, T. et al. Individual risk attitudes: measurement, determinants, and behavioral consequences. J. Eur. Economic Assoc. 9, 522–550 (2011).
Cardon, L. R. & Palmer, L. J. Population stratification and spurious allelic association. Lancet 361, 598–604 (2003).
Nave, G., Jung, W. H., Karlsson Linnér, R., Kable, J. W. & Koellinger, P. D. Are bigger brains smarter? Evidence from a large-scale preregistered study. Psychol. Sci. 30, 43–54 (2019).
Romer, D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev. Psychobiol. 52, 263–276 (2010).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Harper, C. The neurotoxicity of alcohol. Hum. Exp. Toxicol. 26, 251–257 (2007).
Daviet, R. et al. Multimodal brain imaging study of 36,678 participants reveals adverse effects of moderate drinking. Preprint at bioRxiv https://doi.org/10.1101/2020.03.27.011791 (2020).
Kranzler, H. R. et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am. J. Psychiatry 171, 445–452 (2014).
Ashburner, J. & Friston, K. J. Voxel-based morphometry—the methods. Neuroimage 11, 805–821 (2000).
Botvinik-Nezer, R. et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 (2020).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424 (2018).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Hill, W. D. et al. Molecular genetic contributions to social deprivation and household income in UK Biobank. Curr. Biol. 26, 3083–3089 (2016).
Masouleh, S. K., Eickhoff, S. B., Hoffstaedter, F., Genon, S. & The Alzheimer’s Disease NeuroImaging Initiative. Empirical examination of the replicability of associations between brain structure and psychological variables. eLife 8, e43464 (2019).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Mohr, P. N. C., Biele, G. & Heekeren, H. R. Neural processing of risk. J. Neurosci. 30, 6613–6619 (2010).
Wu, C. C., Sacchet, M. D. & Knutson, B. Toward an affective neuroscience account of financial risk taking. Front. Neurosci. 6, 159 (2012).
Schonberg, T., Fox, C. R. & Poldrack, R. A. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci. 15, 11–19 (2011).
Kable, J. W. & Levy, I. Neural markers of individual differences in decision-making. Curr. Opin. Behav. Sci. 5, 100–107 (2015).
Chang, L. J., Yarkoni, T., Khaw, M. W. & Sanfey, A. G. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex 23, 739–749 (2013).
Evans, B. E., Greaves-Lord, K., Euser, A. S., Franken, I. H. A. & Huizink, A. C. The relation between hypothalamic-pituitary-adrenal (HPA) axis activity and age of onset of alcohol use. Addiction 107, 312–322 (2012).
Kreek, M. J., Nielsen, D. A., Butelman, E. R. & LaForge, K. S. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci. 8, 1450–1457 (2005).
O’Doherty, J. et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304, 452–454 (2004).
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L. & Petersen, S. E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 12, 99–105 (2008).
Ivanov, I., Schulz, K. P., London, E. D. & Newcorn, J. H. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am. J. Drug Alcohol Abus. 34, 239–258 (2008).
Heilman, R. M., Crişan, L. G., Houser, D., Miclea, M. & Miu, A. C. Emotion regulation and decision making under risk and uncertainty. Emotion 10, 257–265 (2010).
Tobler, P. N., Christopoulos, G. I., O’Doherty, J. P., Dolan, R. J. & Schultz, W. Risk-dependent reward value signal in human prefrontal cortex. Proc. Natl Acad. Sci. USA 106, 7185–7190 (2009).
Huizink, A. C., Ferdinand, R. F., Ormel, J. & Verhulst, F. C. Hypothalamic-pituitary-adrenal axis activity and early onset of cannabis use. Addiction 101, 1581–1588 (2006).
Margittai, Z. et al. Combined effects of glucocorticoid and noradrenergic activity on loss aversion. Neuropsychopharmacology 43, 334–341 (2018).
Grotzinger, A. D. et al. Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psychol. Sci. 29, 688–699 (2018).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815 (2013).
Mechelli, A., Price, C. J., Friston, K. J. & Ashburner, J. Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 1, 105–113 (2005).
Knoch, D. et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J. Neurosci. 26, 6469–6472 (2006).
BrainSpan Atlas of the Developing Human Brain (Allen Institute for Brain Science, 2015); http://www.brainspan.org/
Loh, P. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
Camerer, C. F. et al. Evaluating the replicability of social science experiments in Nature and Science between 2010 and 2015. Nat. Hum. Behav. 2, 637–644 (2018).
Dickie, D. A. et al. Permutation and parametric tests for effect sizes in voxel-based morphometry of gray matter volume in brain structural MRI. Magn. Reson. Imaging 33, 1299–1305 (2015).
Acknowledgements
This research was carried out under the auspices of the Brain Imaging and Genetics in Behavioural Research (https://big-bear-research.org/) consortium. We thank N.C. Furtner for helpful comments and D. Manfredi for research assistance. The research was conducted using UKB resources under application no. 40830. The study was supported by funding from an National Science Foundation Early Career Development Program grant (no. 1942917) and The Wharton School Dean’s Research fund to G.N., a European Research Council Consolidator grant to P.D.K. (no. 647648 EdGe), and a European Research Council Consolidator grant (no. 725355 BRAINCODES) and a Swiss National Science Foundation grant (no. 100019L_173248) to C.C.R . R.R.W. was financially supported by NIAAA K23 grant (no. K23 AA023894) and H.R.K. was supported by National Institute of Drug Abuse grant no. P30 DA046345. G.N. thanks C. and R. de la Cruz for ongoing support. The work was carried out on the Dutch national e-infrastructure with the support of the SURF Cooperative. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Data can be accessed via the UK Biobank, and data analysis scripts are available on the Open Science Framework (https://osf.io/qkp4g/).
Author information
Authors and Affiliations
Consortia
Contributions
G.A., R.D., J.W.K., P.D.K. and G.N. designed the research plan. G.N., P.D.K. and C.C.R. oversaw the study. G.A., R.D. and R.K.L. analysed the data with critical input from P.D.K., G.N. and C.C.R. G.A., G.N. and P.D.K. wrote the paper and Supplementary Materials. T.A.H., H.R.K. and R.R.W. contributed to and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.R.K. is a member of an advisory board for Dicerna Pharmaceuticals; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was sponsored in the past three years by AbbVie, Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka and Pfizer; and is named as an inventor on PCT patent application no. 15/878,640, entitled ‘Genotype-guided dosing of opioid agonists’ and filed 24 January 2018. All other authors declare no competing interests.
Additional information
Peer review information Nature Human Behaviour thanks Michelle Luciano, Agnieszka Tymula and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Marike Schiffer.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
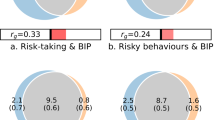
Bivariate correlations between variables used in the main study sample (N = 12,675).
Extended Data Fig. 2
Empirical distributions of variables in the replication sample (N = 13,004).
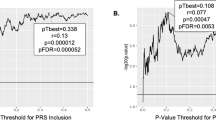
Extended Data Fig. 3 Effect sizes (standardized betas) of associations between risky behaviour and grey matter volume (GMV) in voxel clusters showing significant associations at P < .01 (FWE-corrected) (N = 12,675).
Coordinates of peak association for each cluster are reported in parentheses (in mm). Standard errors denote uncorrected 95% confidence intervals. See Extended Data Table 4 for further details.
Extended Data Fig. 4 Effect sizes (standardized betas) of association between risky behaviour and IDPs of grey matter volume (GMV) showing significant associations at P<0.01 level (FWE-corrected) (N = 12,675).
Standard errors denote uncorrected 95% confidence intervals.
Extended Data Fig. 5 Associations (p-values) between risky behaviour and 148 ROI-level imaging-derived phenotypes (IDPs) of grey matter volume (GMV), controlling for cognitive and socioeconomic outcomes (N = 11,864).
Control variables include education years, fluid IQ, zip-code level social deprivation, household income, number of household members, birth location, and all standard controls.
Extended Data Fig. 6
Associations (p-values) between risky behaviour and 148 ROI-level imaging-derived phenotypes (IDPs) of grey matter volume (GMV), controlling for current drinking levels (binned in deciles) and smoking levels (binned in 3 categories) in addition to all standard controls (N = 12,675).
Extended Data Fig. 7 Meta-analysis of functional MRI studies of risky behaviours, provided by Neurosynth (N = 4,717 participants and K = 101 studies).
Conjunction with areas showing negative GMV association with risky behaviour (including thalamus, vmPFC, amygdala and dlPFC) is marked in magenta (see Supplementary Table 1). Additional meta-analytic functional activation areas are marked in red.
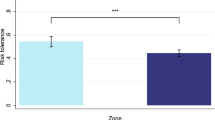
Extended Data Fig. 8 Mediation analysis of the association between PRS and risky behaviour with GMV in dlPFC, putamen and hypothalamus (N = 12,675).
The sum of all GMV differences in right dlPFC, putamen and hypothalamus (based on the activation masks from Fig 5A) mediated ~2.07% of the association between the PRS and risky behaviour. Arrows depict the direction of the structural equation modelling and do not imply causality.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Discussion, Supplementary Tables 1–9, Supplementary References and Supplementary Notes.
Rights and permissions
About this article
Cite this article
Aydogan, G., Daviet, R., Karlsson Linnér, R. et al. Genetic underpinnings of risky behaviour relate to altered neuroanatomy. Nat Hum Behav 5, 787–794 (2021). https://doi.org/10.1038/s41562-020-01027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-020-01027-y
This article is cited by
-
The genetic architecture of the human hypothalamus and its involvement in neuropsychiatric behaviours and disorders
Nature Human Behaviour (2024)
-
Dimensional and transdiagnostic phenotypes in psychiatric genome-wide association studies
Molecular Psychiatry (2023)
-
The critical role of the orbitofrontal cortex for regret in an economic decision-making task
Brain Structure and Function (2022)