Abstract
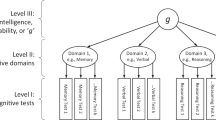
It has been known since 1904 that, in humans, diverse cognitive traits are positively intercorrelated. This forms the basis for the general factor of intelligence (g). Here, we directly test whether there is a partial genetic basis for individual differences in g using data from seven different cognitive tests (n = 11,263–331,679) and genome-wide autosomal single-nucleotide polymorphisms. A genetic g factor accounts for an average of 58.4% (s.e. = 4.8%) of the genetic variance in the cognitive traits considered, with the proportion varying widely across traits (range, 9–95%). We distil genetic loci that are broadly relevant for many cognitive traits (g) from loci associated specifically with individual cognitive traits. These results contribute to elucidating the aetiology of a long-known yet poorly understood phenomenon, revealing a fundamental dimension of genetic sharing across diverse cognitive traits.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Complete summary GWAS results from this paper are available at https://www.lothianbirthcohort.ed.ac.uk/content/summary-data-and-other-resources. Raw data for UKB can be requested at https://www.ukbiobank.ac.uk/register-apply/. Raw data for Generation Scotland can be requested at https://www.ed.ac.uk/generation-scotland/for-researchers/access-to-resources/access.
Code availability
Code to perform common factor modelling and multivariate GWAS within Genomic SEM can be found at https://github.com/MichelNivard/GenomicSEM/wiki.
References
Deary, I. J., Strand, S., Smith, P. & Fernandes, C. Intelligence and educational achievement. Intelligence 35, 13–21 (2007).
Strenze, T. Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence 35, 401–426 (2007).
Calvin, C. M. et al. Childhood intelligence in relation to major causes of death in 68 year follow-up: prospective population study. BMJ 357, j2708 (2017).
Spearman, C. ‘General intelligence,’ objectively determined and measured. Am. J. Psychol. 15, 201–292 (1904).
Carroll, J. B. in Human Cognitive Abilities: A Survey of Factor-Analytic Studies (Cambridge Univ. Press, 1993).
Johnson, W., te Nijenhuis, J. & Bouchard, T. J. Still just 1 g: consistent results from five test batteries. Intelligence 36, 81–95 (2008).
Tucker-Drob, E. M. Differentiation of cognitive abilities across the life span. Dev. Psychol. 45, 1097–1118 (2009).
Cox, S. R., Ritchie, S. J., Fawns-Ritchie, C., Tucker-Drob, E. M. & Deary, I. J. Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76, 101376 (2019).
Deary, I. J. Looking Down on Human Intelligence: From Psychometrics to the Brain (Cambridge Univ. Press, 2000).
Deary, I. J., Penke, L. & Johnson, W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 11, 201–211 (2010).
Haier, R. J. The Neuroscience of Intelligence (Cambridge Univ. Press, 2016).
Kovas, Y. & Plomin, R. Generalist genes: implications for the cognitive sciences. Trends Cogn. Sci. 10, 198–203 (2006).
Plomin, R. & Deary, I. J. Genetics and intelligence differences: five special findings. Mol. Psychiatry 20, 98–108 (2015).
Engelhardt, L. E. et al. Strong genetic overlap between executive functions and intelligence. J. Exp. Psychol. Gen. 145, 1141–1159 (2016).
Panizzon, M. S. et al. Genetic and environmental influences on general cognitive ability: is g a valid latent construct? Intelligence 43, 65–76 (2014).
Petrill, S. A. Molarity versus modularity of cognitive functioning? A behavioral genetic perspective. Curr. Dir. Psychol. Sci. https://doi.org/10.1111/1467-8721.ep11512833 (1997).
Petrill, S. A. et al. The genetic and environmental relationship between general and specific cognitive abilities in twins age 80 and older. Psychol. Sci. https://doi.org/10.1111/1467-9280.00035 (1998).
Rijsdijk, F. V., Vernon, P. A. & Boomsma, D. I. Application of hierarchical genetic models to Raven and WAIS subtests: a Dutch twin study. Behav. Genet. 32, 199–210 (2002).
Davies, G. et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9, 2098 (2018).
Hill, W. D. et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 24, 169–181 (2019).
Savage, J. E. et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919 (2018).
Davies, G. et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112,151). Mol. Psychiatry 21, 758–767 (2016).
Debette, S. et al. Genome-wide studies of verbal declarative memory in nondemented older people: The Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Biol. Psychiatry https://doi.org/10.1016/j.biopsych.2014.08.027 (2015).
Schmidt, F. L. Beyond questionable research methods: the role of omitted relevant research in the credibility of research. Arch. Sci. Psychol. 5, 32–41 (2017).
Grotzinger, A. D. et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 3, 513–525 (2019).
Fawns-Ritchie, C. & Deary, I. J. Reliability and validity of the UK Biobank cognitive tests. PloS ONE 15, e0231627 (2020).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Lee, S. H., Yang, J., Goddard, M. E., Visscher, P. M. & Wray, N. R. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 28, 2540–2542 (2012).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2010.11.011 (2011).
Marioni, R. E. et al. GWAS on family history of Alzheimer’s disease. Transl. Psychiatry 8, 99 (2018).
Hagenaars, S. P. et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112 151) and 24 GWAS consortia. Mol. Psychiatry 21, 1624–1632 (2016).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Turley, P. et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 50, 229–237 (2018).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
Lee, P. H. et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell https://doi.org/10.1016/j.cell.2019.11.020 (2019).
Demontis, D. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75 (2019).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits (n = 17,706). Mol. Psychiatry https://doi.org/10.1038/s41380-019-0569-z (2019).
Timmers, P. R. et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. eLife 8, e39856 (2019).
Anttila, V. et al. Analysis of shared heritability in common disorders of the brain. Science 360, 6395 (2018).
Smith, B. H. et al. Generation Scotland: The Scottish Family Health Study; a new resource for researching genes and heritability. BMC Med. Genet. 7, 74 (2006).
Smith, B. H. et al. Cohort profile: Generation Scotland: Scottish Family Health Study (GS: SFHS). The study, its participants and their potential for genetic research on health and illness. Int. J. Epidemiol. 42, 689–700 (2013).
Jensen, A. R. Clocking the mind: mental chronometry and individual differences. Pers. Psychol. https://doi.org/10.1111/j.1744-6570.2008.00111_7.x (2008).
Demange, P. A. et al. Investigating the genetic architecture of non-cognitive skills using GWAS-by-subtraction. Preprint at bioRxiv https://doi.org/10.1101/2020.01.14.905794 (2020).
Kovacs, K. & Conway, A. R. A. process overlap theory: a unified account of the general factor of intelligence. Psychol. Inq. 27, 151–177 (2016).
Bartholomew, D. J., Deary, I. J. & Lawn, M. A new lease of life for Thomson’s bonds model of intelligence. Psychol. Rev. 116, 567–579 (2009).
Ceci, S. J. On Intelligence–More or Less: A Bio-ecological Treatise on Intellectual Development (Prentice Hall, 1990).
Van Der Maas, H. L. J. et al. A dynamical model of general intelligence: the positive manifold of intelligence by mutualism. Psychol. Rev. 113, 842–861 (2006).
Dickens, W. T. What is g? Brookings Rev. https://www.brookings.edu/research/what-is-g/ (2007).
Tucker-Drob, E. M., Brandmaier, A. M. & Lindenberger, U. Coupled cognitive changes in adulthood: a meta-analysis. Psychol. Bull. 145, 273–301 (2019).
Karen, R. et al. COGNITO: computerized assessment of information processing. J. Psychol. Psychother. https://doi.org/10.4172/2161-0487.1000136 (2014).
Lyall, D. M. et al. Cognitive test scores in UK biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS ONE 11, e0156366 (2016).
Reitan, R. M. & Wolfson, D. The Halstead–Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation Vol. 4 (Neuropsychology Press, 1985).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Rosseel, Y. Lavaan: an R package for structural equation modeling. J. Stat. Softw. https://doi.org/10.18637/jss.v048.i02 (2012).
Hu, L. T. & Bentler, P. M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Modeling 6, 1–55 (1999).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Pe’er, I., Yelensky, R., Altshuler, D. & Daly, M. J. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet. Epidemiol. 32, 381–385 (2008).
Watanabe, K., Taskesen, E., Van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Kerr, S. M. et al. Pedigree and genotyping quality analyses of over 10,000 DNA samples from the Generation Scotland: Scottish Family Health Study. BMC Med. Genet. 14, 38 (2013).
Euesden, J., Lewis, C. M. & O’Reilly, P. F. PRSice: polygenic risk score software. Bioinformatics 31, 1466–1468 (2015).
Acknowledgements
This article benefited from valuable discussions with M. Nivard. This work was supported by National Institutes of Health (NIH) grant no. R01AG054628. The Population Research Center at the University of Texas is supported by NIH grant no. P2CHD042849. I.J.D. and G.D. are with the Lothian Birth Cohorts group, which is funded by Age UK (Disconnected Mind grant), the Medical Research Council (grant no. MR/R024065/1) and the University of Edinburgh’s School of Philosophy, Psychology and Language Sciences. This research was conducted using the UK Biobank Resource (Application Nos. 10279 and 4844). We are grateful for the availability of data from Generation Scotland: Scottish Family Health Study. Generation Scotland received core support from the Chief Scientist Office of the Scottish Government Health Directorates (no. CZD/16/6) and the Scottish Funding Council (no. HR03006). Genotyping of the GS: Scottish Family Health Study samples was carried out by the Genetics Core Laboratory at the Edinburgh Clinical Research Facility, University of Edinburgh, UK and was funded by the Medical Research Council UK and the Wellcome Trust (Wellcome Trust Strategic Award, Stratifying Resilience and Depression Longitudinally, no. 104036/Z/14/Z). The funders had no role in study design, data analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
I.J.D. and E.M.T.-D. jointly conceived of the idea, designed the study and formulated the analytic plan. J.F. and G.D. performed the analyses, with contributions from A.D.G. I.J.D. and E.M.T.-D. wrote the paper, with contributions from J.F. and G.D. All authors contributed to editing the paper.
Corresponding authors
Ethics declarations
Competing interests
I.J.D. is a participant in UKB. All other authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Charlotte Payne.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12, Supplementary Tables 1–6 and Supplementary References.
Supplementary Table
Supplementary Tables 7–30.
Rights and permissions
About this article
Cite this article
de la Fuente, J., Davies, G., Grotzinger, A.D. et al. A general dimension of genetic sharing across diverse cognitive traits inferred from molecular data. Nat Hum Behav 5, 49–58 (2021). https://doi.org/10.1038/s41562-020-00936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-020-00936-2
This article is cited by
-
Beyond the factor indeterminacy problem using genome-wide association data
Nature Human Behaviour (2024)
-
Investigation of genetic determinants of cognitive change in later life
Translational Psychiatry (2024)
-
Genome-wide association study of population-standardised cognitive performance phenotypes in a rural South African community
Communications Biology (2023)
-
Mapping the cortico-striatal transcriptome in attention deficit hyperactivity disorder
Molecular Psychiatry (2023)
-
Multivariate genomic architecture of cortical thickness and surface area at multiple levels of analysis
Nature Communications (2023)