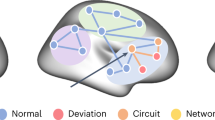
Abstract
Most psychopathological disorders develop in adolescence. The biological basis for this development is poorly understood. To enhance diagnostic characterization and develop improved targeted interventions, it is critical to identify behavioural symptom groups that share neural substrates. We ran analyses to find relationships between behavioural symptoms and neuroimaging measures of brain structure and function in adolescence. We found two symptom groups, consisting of anxiety/depression and executive dysfunction symptoms, respectively, that correlated with distinct sets of brain regions and inter-regional connections, measured by structural and functional neuroimaging modalities. We found that the neural correlates of these symptom groups were present before behavioural symptoms had developed. These neural correlates showed case–control differences in corresponding psychiatric disorders, depression and attention deficit hyperactivity disorder in independent clinical samples. By characterizing behavioural symptom groups based on shared neural mechanisms, our results provide a framework for developing a classification system for psychiatric illness that is based on quantitative neurobehavioural measures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The IMAGEN data used in this investigation will be made available on reasonable request to the corresponding author. All other data are available on reasonable request to the appropriate study leader.
Code availability
The core code used to run the analyses reported in this study are available as Supplementary Software. The supporting code can be found at: https://github.com/alexjamesing/mscca-regression-code.
References
Kessler, R. C. et al. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiat. 20, 359–364 (2007).
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863 (1999).
Steinberg, L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr. Dir. Psychol. Sci. 16, 55–59 (2007).
Gogtay, N. et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl Acad. Sci. USA 101, 8174–8179 (2004).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 23, 28–38 (2016).
Insel, T. et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010).
Lahey, B. B. et al. Is there a general factor of prevalent psychopathology during adulthood? J. Abnorm. Psychol. 121, 971–977 (2012).
Zhang, X. et al. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 113, E6544 (2016).
Rosenberg, M. D. et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 19, 165–171 (2016).
Smith, S. M. et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat. Neurosci. 18, 1565–1567 (2015).
Witten, D. M., Tibshirani, R. & Hastie, T. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics 10, 515–534 (2009).
Xia, C. H. et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 9, 3003 (2018).
Kettenring, J. R. Canonical analysis of several sets of variables. Biometrika 58, 433–451 (1971).
Goodman, R., Ford, T., Richards, H., Gatward, R. & Meltzer, H. The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiat. 41, 645–655 (2000).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007).
Smith, S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 (2006).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Ashburner, J. & Friston, K. J. Voxel-based morphometry—the methods. Neuroimage 11, 805–821 (2000).
Meinshausen, N. & Bühlmann, P. Stability selection. J. R. Stat. Soc. Ser. B 72, 417–473 (2010).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol. Psychiat. 22, 900–909 (2016).
Chen, G. et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci. Rep. 6, 21825 (2016).
Guo, W. et al. Increased cerebellar-default-mode-network connectivity in drug-naive major depressive disorder at rest. Medicine 94, e560 (2015).
Carmona, S. et al. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci. Lett. 389, 88–93 (2005).
Power, J. D., Fair, D. A., Schlaggar, B. L. & Petersen, S. E. The development of human functional brain networks. Neuron 67, 735–748 (2010).
Krueger, R. F., Caspi, A., Moffitt, T. E. & Silva, P. A. The structure and stability of common mental disorders (DSM-III-R): a longitudinal-epidemiological study. J. Abnorm. Psychol. 107, 216 (1998).
Diedenhofen, B. & Musch, J. Cocor: a comprehensive solution for the statistical comparison of correlations. PloS One 10, e0121945 (2015).
Dunn, O. J. & Clark, V. Correlation coefficients measured on the same individuals. J. Am. Stat. Assoc. 64, 366–377 (1969).
Whelan, R. et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature 512, 185–189 (2014).
Lahey, B. B., Van Hulle, C. A., Singh, A. L., Waldman, I. D. & Rathouz, P. J. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch. Gen. Psychiat. 68, 181–189 (2011).
Kessler, R. C. et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s world mental health survey initiative. World Psychiat. 6, 168–176 (2007).
Mayberg, H. S. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med Bull. 65, 193–207 (2003).
Witelson, S. F. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 112, 799–835 (1989).
Tham, M. W., San Woon, P., Sum, M. Y., Lee, T. & Sim, K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J. Affect Disord. 132, 26–36 (2011).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. 98, 676–682 (2001).
Buckner, R. L., Andrews‐Hanna, J. R. & Schacter, D. L. The brain’s default network. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008).
Ray, R. D. et al. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 5, 156–168 (2005).
Stoodley, C. J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11, 352–365 (2012).
Guggenmos, M. et al. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl. Psychiat. 7, 1279–1286 (2017).
Hibar, D. P. et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Mol. Psychiat. 23, 932–942 (2017).
McGorry, P. D., Hickie, I. B., Yung, A. R., Pantelis, C. & Jackson, H. J. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust. N. Z. J. Psychiatry 40, 616–622 (2006).
Biswal, B., Zerrin Yetkin, F., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Schumann, G. et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiat. 15, 1128–1139 (2010).
Goodman, R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiat. 38, 581–586 (1997).
Vulser, H. et al. Subthreshold depression and regional brain volumes in young community adolescents. J. Am. Acad. Child Adolesc. Psychiat. 54, 832–840 (2015).
Kurth, F. & Lüders, E. VBM8. http://www.neuro.uni-jena.de/vbm/download/ (2010).
The FIL Methods Group. SPM8. https://www.fil.ion.ucl.ac.uk/spm/software/spm8/ (2009).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851 (2005).
Grellmann, C. et al. Comparison of variants of canonical correlation analysis and partial least squares for combined analysis of MRI and genetic data. Neuroimage 107, 289–310 (2015).
Jones, D. K. et al. Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum. Brain Mapp. 15, 216–230 (2002).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S219 (2004).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
Pruim, R. H. et al. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
Pruim, R. H., Mennes, M., Buitelaar, J. K. & Beckmann, C. F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112, 278–287 (2015).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 (2007).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Hotelling, H. Relations between two sets of variates. Biometrika 28, 321–377 (1936).
Witten, D. M. & Tibshirani, R. J. Extensions of sparse canonical correlation analysis with applications to genomic data. Stat. Appl. Genet. Mol. Biol. 8, 1–27 (2009).
Parkhomenko, E., Tritchler, D. & Beyene, J. Sparse canonical correlation analysis with application to genomic data integration. Stat. Appl. Genet. Mol. Biol. 8, 1–34 (2009).
Gifi, A. Nonlinear Multivariate Analysis (Wiley, 1990).
Jenkins, L. M. et al. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. NeuroImage Clin. 12, 1022–1034 (2016).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiat. 72, 305–315 (2015).
Everitt, B. S. & Dunn, G. Applied Multivariate Data Analysis Vol 2 (Arnold, 2001).
Timm, N. H. & Carlson, J. E. Part and bipartial canonical correlation analysis. Psychometrika 41, 159–176 (1976).
O’Brien, L. M. et al. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. Neuroimag. 193, 113–122 (2011).
Pell, G. S. et al. Selection of the control group for VBM analysis: influence of covariates, matching and sample size. Neuroimage 41, 1324–1335 (2008).
Voevodskaya, O. et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front. Aging Neurosci. 6, 264 (2014).
Van Den, Wollenberg & Arnold, L. Redundancy analysis: an alternative for canonical correlation analysis. Psychometrika 42, 207–219 (1977).
Stewart, D. & Love, W. A general canonical correlation index. Psychol. Bull. 70, 160–163 (1968).
Monteiro, J. M., Rao, A., Shawe-Taylor, J. & Mourão-Miranda, J. Alzheimer’s Disease Initiative. A multiple hold-out framework for sparse partial least squares. J. Neurosci. Methods 271, 182–194 (2016).
Holmes, A. P., Blair, R. C., Watson, G. & Ford, I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow. Metab. 16, 7–22 (1996).
Westfall, P. H. & Troendle, J. F. Multiple testing with minimal assumptions. Biometrical J. 50, 745–755 (2008).
Westfall, P. H. & Young, S. S. Resampling-based Multiple Testing: Examples and Methods for P-value Adjustment Vol. 279 (Wiley, 1993).
Friedman, J., Hastie, T. & Tibshirani, R. The Elements of Statistical Learning. Springer Series in Statistics, Vol. 1 (Springer, 2001).
Aebi, M. et al. The use of the development and well-being assessment (DAWBA) in clinical practice: a randomized trial. Eur. Child Adolesc. Psychiat. 21, 559–567 (2012).
Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. Regul. Ed. 9, 69–74 (2005).
Schmaal, L. et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Mol. Psychiat. 21, 806 (2016).
Rimol, L. M. et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiat. 71, 552–560 (2012).
van Erp, T. G. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiat. 21, 547 (2016).
von Rhein, D. et al. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. design and descriptives. Eur. Child Adolesc. Psychiat. 24, 265–281 (2015).
Hoogman, M. et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiat. 4, 310–319 (2017).
Acknowledgements
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (reinforcement-related behaviour in normal brain function and psychopathology; grant no. LSHM-CT-2007-037286), the Horizon 2020-funded ERC Advanced Grant STRATIFY (brain-network-based stratification of reinforcement-related disorders; grant no. 695313), ERANID (understanding the interplay between cultural, biological and subjective factors in drug use pathways; grant no. PR-ST-0416-10004), BRIDGET (JPND: brain imaging, cognition, dementia and next generation genomics; grant no. MR/N027558/1), the Human Brain Project (HBP SGA 2, grant no. 785907), the FP7 project MATRICS (grant no. 603016), the Medical Research Council Grant c-VEDA (Consortium on Vulnerability to Externalizing Disorders and Addictions; grant no. MR/N000390/1), the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministerium für Bildung und Forschung (BMBF grant nos. 01GS08152 and 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B, 01ZX1314G, 01GS08147), the Deutsche Forschungsgemeinschaft (DFG grant nos. SM 80/7-2, SFB 940/2), the Medical Research Foundation and Medical Research Council (grant nos. MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH)-funded ENIGMA (grant nos. 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from: ANR (project AF12-NEUR0008-01-WM2NA and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the NIH, Science Foundation Ireland (grant no. 16/ERCD/3797), USA (Axon, Testosterone and Mental Health during Adolescence; grant no. RO1 MH085772-01A1) and NIH Consortium grant no. U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. A.M. gratefully acknowledges funding from the Netherlands Organization for Scientific Research via the Vernieuwingsimpuls VIDI programme (grant no. 016.156.415). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
A.I., C.C., I.M.V., P.G.S., H.L., T.J. and G.R. preprocessed the data. A.I. and P.G.S. analysed the data. A.I., G.S., F.B. and P.G.S. wrote the manuscript. A.I., G.S., T.W.R., A.M., J.A. and E.B. conceptualized the study. N.T., E.B.Q., T.W., S.D., T.B., A.L.W.B., U.B., C.B., P.C., T.F., H.F., V.F., H.G., P.S., P.G., Y.G., A.H., B.I., V.K., J.-L.M., A.M.-L., F.N., B.v.N., D.P.O., M.-L.P.M., S.M., J.P., L.P., M.S., A.S., M.N.S., H.W., R.W., O.A.A., I.A., E.D.B. and J.B. collected data. A.I. and N.T. prepared the figures. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.B. served in an advisory or consultancy role for Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH and Shire. He received conference support or a speaker’s fee from Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire and Viforpharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press. The present work is unrelated to the above grants and relationships. E.D.B. received honoraria from General Electric Healthcare for teaching on scanner programming courses and acts as a consultant for IXICO. O.A.A. received a speaker’s honorarium from Lundbeck. G.R. received financial support from scientific meetings (Janssen & Janssen, Otsuka−Lundbeck). A.M.-L. received consultant fees from Boehringer Ingelheim, Brainsway, Elsevier, Lundbeck Int. Neuroscience Foundation and Science Advances. The other authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Mary Elizabeth Sutherland.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figs. 1−10, and Tables 1−9, and Supplementary Note (containing the list of authors for the IMAGEN Consortium).
Supplementary Software
This msCCA script forms the basis of the msCCA-regression approach used in the present investigation.
Rights and permissions
About this article
Cite this article
Ing, A., Sämann, P.G., Chu, C. et al. Identification of neurobehavioural symptom groups based on shared brain mechanisms. Nat Hum Behav 3, 1306–1318 (2019). https://doi.org/10.1038/s41562-019-0738-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-019-0738-8
This article is cited by
-
Das Deutsche Zentrum für Psychische Gesundheit
Der Nervenarzt (2024)
-
Neurodevelopmental risk and adaptation as a model for comorbidity among internalizing and externalizing disorders: genomics and cell-specific expression enriched morphometric study
BMC Medicine (2023)
-
Challenges and future directions for investigating the effects of urbanicity on mental health
Nature Mental Health (2023)
-
Nighttime lights, urban features, household poverty, depression, and obesity
Current Psychology (2023)
-
Functional connectivity signatures of major depressive disorder: machine learning analysis of two multicenter neuroimaging studies
Molecular Psychiatry (2023)