Abstract
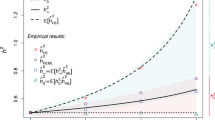
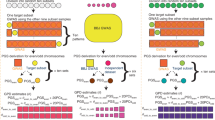
Preference for mates with similar phenotypes; that is, assortative mating, is widely observed in humans1,2,3,4,5 and has evolutionary consequences6,7,8. Under Fisher's classical theory6, assortative mating is predicted to induce a signature in the genome at trait-associated loci that can be detected and quantified. Here, we develop and apply a method to quantify assortative mating on a specific trait by estimating the correlation (θ) between genetic predictors of the trait from single nucleotide polymorphisms on odd- versus even-numbered chromosomes. We show by theory and simulation that the effect of assortative mating can be quantified in the presence of population stratification. We applied this approach to 32 complex traits and diseases using single nucleotide polymorphism data from ~400,000 unrelated individuals of European ancestry. We found significant evidence of assortative mating for height (θ = 3.2%) and educational attainment (θ = 2.7%), both of which were consistent with theoretical predictions. Overall, our results imply that assortative mating involves multiple traits and affects the genomic architecture of loci that are associated with these traits, and that the consequence of mate choice can be detected from a random sample of genomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
We used genotypic data from the Resource for Genetic Epidemiology Research on Adult Health and Aging (GERA: dbGaP phs000674.v2.p2), genotypic and phenotypic data from the Health and Retirement Study (HRS: dbGaP phs000428.v1.p1), and genotypic and phenotypic data from the UKB under project 12505.
References
Pearson, K. & Lee, A. On the laws of inheritance in man: I. Inheritance of physical characters. Biometrika 2, 357–462 (1903).
Spuhler, J. N. Assortative mating with respect to physical characteristics. Eugen. Q 15, 128–140 (1968).
Mare, R. D. Five decades of educational assortative mating. Am. Sociol. Rev. 56, 15–32 (1991).
Silventoinen, K., Kaprio, J., Lahelma, E., Viken, R. J. & Rose, R. J. Assortative mating by body height and BMI: Finnish twins and their spouses. Am. J. Hum. Biol. 15, 620–627 (2003).
Stulp, G., Simons, M. J. P., Grasman, S. & Pollet, T. V. Assortative mating for human height: a meta-analysis. Am. J. Hum. Biol 29, e22917 (2016).
Fisher, R. A. The correlation between relatives on the supposition of Mendelian inheritance Trans. R. Soc. Edinb. 52, 399–433 (1918).
Wright, S. Systems of mating. III. Assortative mating based on somatic resemblance. Genetics 6, 144–161 (1921).
Crow, J. F. & Kimura, M. An Introduction to Population Genetics Theory (Blackburn Press, Caldwell, 2009).
Shine, R., O’connor, D., Lemaster, M. P. & Mason, R. T. Pick on someone your own size: ontogenetic shifts in mate choice by male garter snakes result in size-assortative mating. Anim. Behav. 61, 1133–1141 (2001).
Jiang, Y., Bolnick, D. I. & Kirkpatrick, M. Assortative mating in animals. Am. Nat. 181, E125–E138 (2013).
Vandenburg, S. G. Assortative mating, or who marries whom? Behav. Genet. 2, 127–157 (1972).
Hippisley-Cox, J., Coupland, C., Pringle, M., Crown, N. & Hammersley, V. Married couples' risk of same disease: cross sectional study. Br. Med. J. 325, 636 (2002).
Ajslev, T. A. et al. Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Front. Genet. 3, 125 (2012).
Nordsletten, A. E. et al. Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry 73, 354–361 (2016).
Nagylaki, T. Assortative mating for a quantitative character. J. Math. Biol. 16, 57–74 (1982).
Gimelfarb, A. Quantitative characters under assortative mating: gametic model. Theor. Popul. Biol. 25, 312–330 (1984).
Bulmer, M. G. The Mathematical Theory of Quantitative Genetics (Clarendon Press, Oxford, 1985).
Sebro, R., Peloso, G. M., Dupuis, J. & Risch, N. J. Structured mating: patterns and implications. PLoS Genet. 13, e1006655 (2017).
Sebro, R., Hoffman, T. J., Lange, C., Rogus, J. J. & Risch, N. J. Testing for non-random mating: evidence for ancestry-related assortative mating in the Framingham Heart Study. Genet. Epidemiol. 34, 674–679 (2010).
Risch, N. et al. Ancestry-related assortative mating in Latino populations. Genome Biol. 10, R132 (2009).
Crow, J. F. & Felsenstein, J. The effect of assortative mating on the genetic composition of a population. Eugen. Q. 15, 85–97 (1968).
Boyle, E. A., Li, Y. I. & Pritchard, J. K. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 (2017).
Li, X., Redline, S., Zhang, X., Williams, S. & Zhu, X. Height associated variants demonstrate assortative mating in human populations. Sci. Rep. 7, 15689 (2017).
Sampson, J., Kidd, K. K., Kidd, J. R. & Zhao, H. Selecting SNPs to identify ancestry. Ann. Hum. Genet. 75, 539–553 (2011).
Wood, A. R. et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 46, 1173–1186 (2014).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Robinson, M. R. et al. Population genetic differentiation of height and body mass index across Europe. Nat. Genet. 47, 1357–1362 (2015).
Okbay, A. et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533, 539–542 (2016).
Cesarini, D. & Visscher, P. M. Genetics and educational attainment. npj Sci. Learn. 2, 4 (2017).
Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. Nature 562, 203–209 (2018).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Kemp, J. P. et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat. Genet. 49, 1468–1475 (2017).
Robinson, M. R. et al. Genetic evidence of assortative mating in humans. Nat. Hum. Behav 1, 0016 (2017).
Hugh-Jones, D., Verweij, K. J. H., St. Pourcain, B. & Abdellaoui, A. Assortative mating on educational attainment leads to genetic spousal resemblance for polygenic scores. Intelligence 59, 103–108 (2016).
Conley, D. et al. Assortative mating and differential fertility by phenotype and genotype across the 20th century. Proc. Natl Acad. Sci. USA 113, 6647–6652 (2016).
Agrawal, A. et al. Assortative mating for cigarette smoking and for alcohol consumption in female Australian twins and their spouses. Behav. Genet. 36, 553–566 (2006).
Youyou, W., Stillwell, D., Schwartz, H. A. & Kosinski, M. Birds of a feather do flock together: behavior-based personality-assessment method reveals personality similarity among couples and friends. Psychol. Sci. 28, 276–284 (2017).
Berg, J. J. & Coop, G. A population genetic signal of polygenic adaptation. PLoS Genet. 10, e1004412 (2014).
Field, Y. et al. Detection of human adaptation during the past 2000 years. Science 354, 760–764 (2016).
Tenesa, A., Rawlik, K., Navarro, P. & Canela-Xandri, O. Genetic determination of height-mediated mate choice. Genome Biol. 16, 269 (2016).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Eaves, L. J., Last, K. A., Young, P. A. & Martin, N. G. Model-fitting approaches to the analysis of human behaviour. Heredity 41, 249–320 (1978).
Galinsky, K. J. et al. Fast principal-component analysis reveals convergent evolution of ADH1B in Europe and East Asia. Am. J. Hum. Genet. 98, 456–472 (2016).
Lachin, J. M. Introduction to sample size determination and power analysis for clinical trials. Control. Clin. Trials 2, 93–113 (1981).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Allen, N. et al. UK Biobank: current status and what it means for epidemiology. Health Pol. Technol. 1, 123–126 (2012).
International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
1000 Genomes Project Consortium A global reference for human genetic variation. Nature 526, 68–74 (2015).
Sniekers, S. et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat. Genet. 49, 1107–1112 (2017).
Day, F. R. et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat. Genet. 47, 1294–1303 (2015).
Acknowledgements
This research was supported by the Australian Research Council (DP130102666, DP160103860 and DP160102400), Australian National Health and Medical Research Council (1078037, 1078901, 1103418, 1107258, 1127440 and 1113400), National Institutes of Health (grants R01AG042568, P01GM099568 and R01MH100141) and Sylvia and Charles Viertel Charitable Foundation. The GERA study was supported by grant RC2 AG036607 from the National Institutes of Health, and grants from the Robert Wood Johnson Foundation, Ellison Medical Foundation, Wayne and Gladys Valley Foundation and Kaiser Permanente. The authors thank members of the Kaiser Permanente Medical Care Plan, Northern California Region who generously agreed to participate in the Kaiser Permanente Research Program on Genes, Environment and Health. This research has been conducted using the UKB Resource under project 12505. We thank B. Hill for helpful comments and suggestions on the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.M.V., L.Y., M.R.R., J.Y. and M.E.G. conceived and designed the study. L.Y., M.T. and N.R.W. curated the summary statistics. L.Y. and P.M.V. derived the theory. Y.Y., M.T., J.G., K.E.K. and L.Y. performed the mate pairs analyses. M.C.K., P.T., D.J.B. and D.C. helped to develop the methodology and interpret the results. P.M.V., N.R.W., M.R.R. and L.Y. performed the sibling pairs analyses. K.E.K. and L.Y. performed quality control of the UKB data. L.Y. and M.R.R. performed statistical analyses and simulations. L.Y. and P.M.V. wrote the manuscript with the participation of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Supplementary Figures 1–5, Supplementary Notes 1–4
Rights and permissions
About this article
Cite this article
Yengo, L., Robinson, M.R., Keller, M.C. et al. Imprint of assortative mating on the human genome. Nat Hum Behav 2, 948–954 (2018). https://doi.org/10.1038/s41562-018-0476-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0476-3
This article is cited by
-
More than nature and nurture, indirect genetic effects on children’s academic achievement are consequences of dynastic social processes
Nature Human Behaviour (2024)
-
The impact of assortative mating, participation bias and socioeconomic status on the polygenic risk of behavioural and psychiatric traits
Nature Human Behaviour (2024)
-
Genetic similarity between relatives provides evidence on the presence and history of assortative mating
Nature Communications (2024)
-
Genetic basis of STEM occupational choice and regional economic performance: a UK biobank genome-wide association study
Human Genomics (2023)
-
rBahadur: efficient simulation of structured high-dimensional genotype data with applications to assortative mating
BMC Bioinformatics (2023)