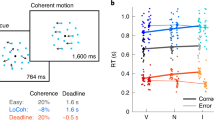
Abstract
Ageing impacts on decision-making behaviour across a range of cognitive tasks and scenarios. Computational modelling has proved valuable in providing mechanistic interpretations of these age-related differences; however, the extent to which model parameter differences accurately reflect changes to the underlying neural computations remains unclear. Here, we report that age-related effects on neural signatures of decision formation are inconsistent with behavioural fits derived from a prominent accumulation-to-bound model. Most notably, model-predicted bound differences were absent neurophysiologically. However, constraining the model to match the decision-predictive elements of the brain signals provided more parsimonious fits to behaviour and generated predictions regarding the neural data that were empirically validated. These included a task-dependent slowing of evidence accumulation among older adults and reduced between-trial accumulation rate variability, which was linked to enhanced attentional engagement. Our findings highlight how combining neurophysiological measurements with computational modelling can yield unique insights into group differences in neural decision mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of the study are available from the corresponding author upon reasonable request.
References
Levine, B., Svoboda, E., Hay, J. F., Winocur, G. & Moscovitch, M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol. Aging 17, 677–689 (2002).
Gazzaley, A., Cooney, J. W., Rissman, J. & D'Esposito, M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 8, 1298–1300 (2005).
Salthouse, T. A. Constraints on theories of cognitive aging. Psychon. Bull. Rev. 3, 287–299 (1996).
Wasylyshyn, C., Verhaeghen, P. & Sliwinski, M. J. Aging and task switching: a meta-analysis. Psychol. Aging 26, 15–20 (2011).
Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460 (2002).
Park, D. C. & Reuter-Lorenz, P. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196 (2009).
Laming, D. R. J. Information Theory of Choice-Reaction Times (Academic Press, London, 1968).
Link, S. W. & Heath, R. A. A sequential theory of psychological discrimination. Psychometrika 40, 77–105 (1975).
Ratcliff, R. A theory of memory retrieval. Psychol. Rev. 85, 59–108 (1978).
Ratcliff, R., Thapar, A. & McKoon, G. The effects of aging on reaction time in a signal detection task. Psychol. Aging 16, 323–341 (2001).
Ratcliff, R., Thapar, A. & McKoon, G. A diffusion model analysis of the effects of aging on brightness discrimination. Percept. Psychophys. 65, 523–535 (2003).
Ratcliff, R., Thapar, A. & McKoon, G. Aging, practice, and perceptual tasks: a diffusion model analysis. Psychol. Aging 21, 353–371 (2006).
Starns, J. J. & Ratcliff, R. The effects of aging on the speed-accuracy compromise: boundary optimality in the diffusion model. Psychol. Aging 25, 377–390 (2010).
Ratcliff, R., Thapar, A. & McKoon, G. Individual differences, aging, and IQ in two-choice tasks. Cogn. Psychol. 60, 127–157 (2010).
Spaniol, J., Voss, A. & Grady, C. L. Aging and emotional memory: cognitive mechanisms underlying the positivity effect. Psychol. Aging 23, 859–872 (2008).
Forstmann, B. U. et al. The speed-accuracy tradeoff in the elderly brain: a structural model-based approach. J. Neurosci. 31, 17242–17249 (2011).
Rabbitt, P. How old and young subjects monitor and control responses for accuracy and speed. Br. J. Psychol. 70, 305–311 (1979).
Ratcliff, R., Thapar, A., Gomez, P. & McKoon, G. A diffusion model analysis of the effects of aging in the lexical-decision task. Psychol. Aging 19, 278–289 (2004).
Ratcliff, R., Thapar, A. & McKoon, G. Aging and individual differences in rapid two-choice decisions. Psychon. Bull. Rev. 13, 626–635 (2006).
Ratcliff, R., Thapar, A. & McKoon, G. Application of the diffusion model to two-choice tasks for adults 75–90 years old. Psychol. Aging 22, 56–66 (2007).
Thapar, A., Ratcliff, R. & McKoon, G. A diffusion model analysis of the effects of aging on letter discrimination. Psychol. Aging 18, 415–429 (2003).
Ratcliff, R., Thapar, A. & McKoon, G. Effects of aging and IQ on item and associative memory. J. Exp. Psychol. Gen. 140, 464–487 (2011).
Dully, J., McGovern, D. P. & O'Connell, R. G. The impact of natural aging on computational and neural indices of perceptualdecision making: a review. Behav. Brain Res. 355, 48–55 (2018).
Robertson, I. H. A noradrenergic theory of cognitive reserve: implications for Alzheimer's disease. Neurobiol. Aging 34, 298–308 (2013).
Hanks, T., Kiani, R. & Shadlen, M. N. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. Elife 3, e02260 (2014).
Hanks, T. D., Mazurek, M. E., Kiani, R., Hopp, E. & Shadlen, M. N. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J. Neurosci. 31, 6339–6352 (2011).
Heitz, R. P. & Schall, J. D. Neural chronometry and coherency across speed-accuracy demands reveal lack of homomorphism between computational and neural mechanisms of evidence accumulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130071 (2013).
Purcell, B. A. & Kiani, R. Neural mechanisms of post-error adjustments of decision policy in parietal cortex. Neuron 89, 658–671 (2016).
Hanes, D. P. & Schall, J. D. Neural control of voluntary movement initiation. Science 274, 427–430 (1996).
Roitman, J. D. & Shadlen, M. N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 22, 9475–9489 (2002).
Ratcliff, R., Hasegawa, Y. T., Hasegawa, R. P., Smith, P. L. & Segraves, M. A. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J. Neurophysiol. 97, 1756–1774 (2007).
Kelly, S. P. & O'Connell, R. G. Internal and external influences on the rate of sensory evidence accumulation in the human brain. J. Neurosci. 33, 19434–19441 (2013).
O’Connell, R. G., Dockree, P. M. & Kelly, S. P. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat. Neurosci. 15, 1729–1735 (2012).
de Lange, F. P., Rahnev, D. A., Donner, T. H. & Lau, H. Prestimulus oscillatory activity over motor cortex reflects perceptual expectations. J. Neurosci. 33, 1400–1410 (2013).
Donner, T. H., Siegel, M., Fries, P. & Engel, A. K. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr. Biol. 19, 1581–1585 (2009).
Murphy, P. R., Boonstra, E. & Nieuwenhuis, S. Global gain modulation generates time-dependent urgency during perceptual choice in humans. Nat. Commun. 7, 13526 (2016).
Twomey, D. M., Kelly, S. P. & O’Connell, R. G. Abstract and effector-selective decision signals exhibit qualitatively distinct dynamics before delayed perceptual reports. J. Neurosci. 36, 7346–7352 (2016).
Ball, K. & Sekuler, R. Improving visual perception in older observers. J. Gerontol. 41, 176–182 (1986).
Billino, J., Bremmer, F. & Gegenfurtner, K. R. Differential aging of motion processing mechanisms: evidence against general perceptual decline. Vision Res. 48, 1254–1261 (2008).
Loughnane, G. M. et al. Target selection signals influence perceptual decisions by modulating the onset and rate of evidence accumulation. Curr. Biol. 26, 496–502 (2016).
Steinemann, N. A., O’Connell, R. G. & Kelly, S. P. Decisions are expedited through multiple neural adjustments spanning the sensorimotor hierarchy.Nat. Commun. 9, 3627 (2018).
Jepma, M., Wagenmakers, E. J. & Nieuwenhuis, S. Temporal expectation and information processing: a model-based analysis. Cognition 122, 426–441 (2012).
Ratcliff, R. & Van Dongen, H. P. A. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proc. Natl Acad. Sci. USA 108, 11285–11290 (2011).
Di Russo, F. et al. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum. Brain Mapp. 28, 323–334 (2007).
Hanslmayr, S. et al. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 37, 1465–1473 (2007).
O’Connell, R. G. et al. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J. Neurosci. 29, 8604–8611 (2009).
Dockree, P. M. et al. The effects of methylphenidate on the neural signatures of sustained attention. Biol. Psychiatry 82, 687–694 (2017).
Murphy, P. R., Vandekerckhove, J. & Nieuwenhuis, S. Pupil-linked arousal determines variability in perceptual decision making. PLoS Comput. Biol. 10, e1003854 (2014).
Turner, B. M., van Maanen, L. & Forstmann, B. U. Informing cognitive abstractions through neuroimaging: the neural drift diffusion model. Psychol. Rev. 122, 312–336 (2015).
Turner, B. M., Forstmann, B. U., Love, B. C., Palmeri, T. J. & Van Maanen, L. Approaches to analysis in model-based cognitive neuroscience. J. Math. Psychol. 76, 65–79 (2017).
Turner, B. M. et al. A Bayesian framework for simultaneously modeling neural and behavioral data. Neuroimage 72, 193–206 (2013).
Turner, B. M., Rodriguez, C. A., Norcia, T. M., McClure, S. M. & Steyvers, M. Why more is better: simultaneous modeling of EEG, fMRI, and behavioral data. Neuroimage 128, 96–115 (2016).
Frank, M. J. et al. fMRI and EEG predictors of dynamic decision parameters during human reinforcement learning. J. Neurosci. 35, 485–494 (2015).
Yang, Y. et al. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience 156, 748–757 (2008).
Yang, Y. et al. Aging affects the neural representation of speed in Macaque area MT. Cereb. Cortex 19, 1957–1967 (2009).
Liang, Z. et al. Aging affects the direction selectivity of MT cells in rhesus monkeys. Neurobiol. Aging 31, 863–873 (2010).
Owsley, C., Sekuler, R. & Siemsen, D. Contrast sensitivity throughout adulthood. Vision Res. 23, 689–699 (1983).
Elliott, D., Whitaker, D. & MacVeigh, D. Neural contribution to spatiotemporal contrast sensitivity decline in healthy ageing eyes. Vision Res. 30, 541–547 (1990).
Habak, C. & Faubert, J. Larger effect of aging on the perception of higher-order stimuli. Vision Res. 40, 943–950 (2000).
Laming, D. Choice reaction performance following an error. Acta Psychol. 43, 199–224 (1979).
Kelly, S. P. & O’Connell, R. G. The neural processes underlying perceptual decision making in humans: recent progress and future directions. J. Physiol. Paris 109, 27–37 (2015).
Afacan-Seref, K., Steinemann, N. A., Blangero, A. & Kelly, S. P. Dynamic interplay of value and sensory information in high-speed decision making. Curr. Biol. 28, 795–802 (2018).
Usher, M. & McClelland, J. L. The time course of perceptual choice: the leaky, competing accumulator model. Psychol. Rev. 108, 550–592 (2001).
Wagenmakers, E. J., van der Maas, H. L. J. & Grasman, R. P. An EZ-diffusion model for response time and accuracy. Psychon. Bull. Rev. 14, 3–22 (2007).
van Ravenzwaaij, D., Donkin, C. & Vandekerckhove, J. The EZ diffusion model provides a powerful test of simple empirical effects. Psychon. Bull. Rev. 24, 547–556 (2017).
Hauser, T. U., Fiore, V. G., Moutoussis, M. & Dolan, R. J. Computational psychiatry of ADHD: neural gain impairments across Marrian levels of analysis. Trends Neurosci. 39, 63–73 (2016).
Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 8, 1458–1463 (2005).
O’Connell, R. G. et al. A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiol. Aging 33, 2448–2461 (2012).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Kayser, J. & Tenke, C. E. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 117, 348–368 (2006).
Silberstein, R. B. et al. Steady-state visually evoked potential topography associated with a visual vigilance task. Brain Topogr. 3, 337–347 (1990).
Silberstein, R. B., Nunez, P. L., Pipingas, A., Harris, P. & Danieli, F. Steady state visually evoked potential (SSVEP) topography in a graded working memory task. Int. J. Psychophysiol. 42, 219–232 (2001).
Barlow, J. S. The Electroencephalogram: Its Patterns and Origins (MIT Press, Cambridge, MA, 1993).
JASP v.0.8.3 (JASP Team, 2017); https://jasp-stats.org/2017/10/10/just-jasp-0-8-3-bang-no-bucks/
Rouder, J. N., Speckman, P. L., Sun, D., Morey, R. D. & Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 16, 225–237 (2009).
Ratcliff, R. & McKoon, G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 20, 873–922 (2008).
Ratcliff, R. & Tuerlinckx, F. Estimating parameters of the diffusion model: approaches to dealing with contaminant reaction times and parameter variability. Psychon. Bull. Rev. 9, 438–481 (2002).
Murphy, P. R., Robertson, I. H., Harty, S. & O'Connell, R. G. Neural evidence accumulation persists after choice to inform metacognitive judgments. eLife 4, e11946 (2015).
Acknowledgements
This work was supported by a European Research Council (ERC) Starting Grant (to R.G.O.C.) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 638289), by a Science Foundation Ireland ERC Support Grant (to R.G.O.C.) and an Irish Research Council Postgraduate Scholarship (to A.H.). S.P.K. is supported by a Career Development Award from Science Foundation Ireland (15/CDA/3591). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
R.G.O.C., S.P.K. and A.H. conceived and designed the experiments. A.H. collected the data. D.P.M. analysed the data and fitted the models. D.P.M., R.G.O.C. and S.P.K. wrote the manuscript; all authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–3
Rights and permissions
About this article
Cite this article
McGovern, D.P., Hayes, A., Kelly, S.P. et al. Reconciling age-related changes in behavioural and neural indices of human perceptual decision-making. Nat Hum Behav 2, 955–966 (2018). https://doi.org/10.1038/s41562-018-0465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0465-6
This article is cited by
-
A General Integrative Neurocognitive Modeling Framework to Jointly Describe EEG and Decision-making on Single Trials
Computational Brain & Behavior (2023)
-
Mental speed is high until age 60 as revealed by analysis of over a million participants
Nature Human Behaviour (2022)
-
A theoretical model of health management using data-driven decision-making: the future of precision medicine and health
Journal of Translational Medicine (2021)
-
Thalamocortical excitability modulation guides human perception under uncertainty
Nature Communications (2021)
-
Auditory information enhances post-sensory visual evidence during rapid multisensory decision-making
Nature Communications (2020)