Abstract
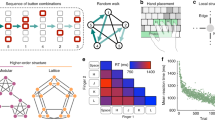
Human learners are adept at grasping the complex relationships underlying incoming sequential input1. In the present work, we formalize complex relationships as graph structures2 derived from temporal associations3,4 in motor sequences. Next, we explore the extent to which learners are sensitive to key variations in the topological properties5 inherent to those graph structures. Participants performed a probabilistic motor sequence task in which the order of button presses was determined by the traversal of graphs with modular, lattice-like or random organization. Graph nodes each represented a unique button press, and edges represented a transition between button presses. The results indicate that learning, indexed here by participants’ response times, was strongly mediated by the graph’s mesoscale organization, with modular graphs being associated with shorter response times than random and lattice graphs. Moreover, variations in a node’s number of connections (degree) and a node’s role in mediating long-distance communication (betweenness centrality) impacted graph learning, even after accounting for the level of practice on that node. These results demonstrate that the graph architecture underlying temporal sequences of stimuli fundamentally constrains learning, and moreover that tools from network science provide a valuable framework for assessing how learners encode complex, temporally structured information.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aslin, R. N. & Newport, E. L. Statistical learning: from acquiring specific items to forming general rules. Curr. Dir. Psychol. Sci. 21, 170–176 (2012).
Newman, M. E. J. Networks: An Introduction (Oxford Univ. Press, Oxford, 2010).
Schapiro, A. C., Rogers, T. T., Cordova, N. I., Turk-Browne, N. B. & Botvinick, M. M. Neural representations of events arise from temporal community structure. Nat. Neurosci. 16, 486–492 (2013).
Karuza, E. A., Kahn, A. E., Thompson-Schill, S. L. & Bassett, D. S. Process reveals structure: how a network is traversed mediates expectations about its architecture. Sci. Rep. 7, 12733 (2017).
Newman, M. E. J. Complex systems: a survey. Am. J. Phys. 79, 800–810 (2011).
Saffran, J. R., Aslin, R. N. & Newport, E. L. Statistical learning by 8-month-old infants. Science 274, 1926–1928 (1996).
Nissen, M. J. & Bullemer, P. Attentional requirements of learning: evidence from performance measures. Cognit. Psychol. 19, 1–32 (1987).
Hunt, R. H. & Aslin, R. N. Statistical learning in a serial reaction time task: access to separable statistical cues by individual learners. J. Exp. Psychol. Gen. 130, 658–680 (2001).
Fiser, J. & Aslin, R. N. Statistical learning of higher-order temporal structure from visual shape sequences. J. Exp. Psychol. Learn. Mem. Cogn. 28, 458–467 (2002).
Turk-Browne, N. B., Jungé, J. A. & Scholl, B. J. The automaticity of visual statistical learning. J. Exp. Psychol. Gen. 134, 552–564 (2005).
Cleeremans, A. & McClelland, J. L. Learning the structure of event sequences. J. Exp. Psychol. Gen. 120, 235–253 (1991).
Furl, N. et al. Neural prediction of higher-order auditory sequence statistics. NeuroImage 54, 2267–2277 (2011).
Newport, E. L. & Aslin, R. N. Learning at a distance I. Statistical learning of non-adjacent dependencies. Cogn. Psychol. 48, 127–162 (2004).
Gómez, R. L. Variability and detection of invariant structure. Psychol. Sci. 13, 431–436 (2002).
Bollobas, B. Random Graphs (Cambridge Univ. Press, Cambridge, 2001).
Jarvis, J. P. & Shier, D. R. in Applied Mathematical Modeling: A Multidisciplinary Approach (eds Shier, D. R. & Wallenius, K. T.) Ch. 13 (Chapman and Hall/CRC Press, Boca Raton, 1999).
Goldstein, R. & Vitevitch, M. S. The influence of clustering coefficient on word-learning: how groups of similar sounding words facilitate acquisition. Front. Psychol. 5, 2009–2014 (2014).
Bales, M. E. & Johnson, S. B. Graph theoretic modeling of large-scale semantic networks. J. Biomed. Inform. 39, 451–464 (2006).
Vitevitch, M. S. What can graph theory tell us about word learning and lexical retrieval? J. Speech. Lang. Hear. Res. 51, 408–422 (2008).
Palla, G., Barabasi, A. L. & Vicsek, T. Quantifying social group evolution. Nature 446, 664–667 (2007).
Girvan, M. & Newman, M. E. J. Community structure in social and biological networks. Proc. Natl Acad. Sci. USA 99, 7821–7826 (2002).
Karuza, E. A., Thompson-Schill, S. L. & Bassett, D. S. Local patterns to global architectures: influences of network topology on human learning. Trends Cogn. Sci. 20, 629–640 (2016).
Steyvers, M. & Tenenbaum, J. B. The large-scale structure of semantic networks: statistical analyses and a model of semantic growth. Cogn. Sci. 29, 41–78 (2005).
Mengistu, H., Huizinga, J., Mouret, J. B. & Clune, J. The evolutionary origins of hierarchy. PLoS Comput. Biol. 12, e1004829 (2016).
Hermundstad, A. M. et al. Variance predicts salience in central sensory processing. eLife 3, e03722 (2014).
Heathcote, A., Brown, S. & Mewhort, D. J. K. The power law repealed: the case for an exponential law of practice. Psychon. Bull. Rev. 7, 185–207 (2000).
Karuza, E. A., Farmer, T. A., Fine, A. B., Smith, F. X. & Jaeger, T. F. On-line measures of prediction in a self-paced statistical learning task. In Proc. 36th Annual Meeting of the Cognitive Science Society (2014).
Cleeremans, A., Destrebecqz, A. & Boyer, M. Implicit learning: news from the front. Trends Cogn. Sci. 2, 406–416 (1998).
Robertson, E. M. The serial reaction time task: implicit motor skill learning? J. Neurosci. 27, 10073–10075 (2007).
Reber, A. S. Implicit learning of artificial grammars. J. Verbal Learning Verbal Behav. 6, 855–863 (1967).
Verwey, W. B., Abrahamse, E. L. & de Kleine, E. Cognitive processing in new and practiced discrete keying sequences. Front. Psychol. 1, 1–13 (2010).
Kiesel, A. et al. Control and interference in task switching—a review. Psychol. Bull. 136, 849–874 (2010).
Koch, I. Automatic and intentional activation of task sets. J. Exp. Psychol. Learn. Mem. Cogn. 27, 1474–1486 (2001).
Gotler, A., Meiran, N. & Tzelgov, J. Nonintentional task set activation: evidence from implicit task sequence learning. Psychon. Bull. Rev. 10, 890–896 (2003).
Schneider, D. W. & Logan, G. D. Hierarchical control of cognitive processes: switching tasks in sequences. J. Exp. Psychol. 135, 623–640 (2006).
Gleiser, P. & Danon, L. Community structure in jazz. Adv. Complex. Syst. 6, 565–573 (2003).
Tompson, S. H., Kahn, A. E., Falk, E. B., Vettel, J. M. & Bassett, D. S. Individual differences in learning social and nonsocial network structures. J. Exp. Psychol. Learn. Mem. Cogn. https://doi.org/10.1037/xlm0000580 (2018).
Gebhart, A. L., Aslin, R. N. & Newport, E. L. Changing structures in midstream: learning along the statistical garden path. Cogn. Sci. 33, 1087–1116 (2009).
Deroost, N. & Soetens, E. Perceptual or motor learning in SRT tasks with complex sequence structures. Psychol. Res. 70, 88–102 (2006).
Messinger, A., Squire, L. R., Zola, S. M. & Albright, T. D. Neuronal representations of stimulus associations develop in the temporal lobe during learning. Proc. Natl Acad. Sci. USA 98, 12239–12244 (2001).
Li, N. & DiCarlo, J. J. Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science 321, 1502–1507 (2008).
Garvert, M. M., Dolan, R. J. & Behrens, T. E. A map of abstract relational knowledge in the human hippocampal-entorhinal cortex. eLife 6, e17086 (2017).
Newman, M. E. Modularity and community structure in networks. Proc. Natl Acad. Sci. USA 103, 8577–8582 (2006).
Acknowledgements
We thank D. M. Lydon-Staley and S. H. Tompson for feedback on earlier versions of this manuscript. This work was supported by the National Science Foundation CAREER award (PHY-1554488 to D.S.B.), Army Research Laboratory through contract number W911NF-10-2-0022 and Army Research Office through contract number Grafton-W911NF-16-1-0474 and contract number DCIST- W911NF-17-2-0181. We also acknowledge additional support from the John D. and Catherine T. MacArthur Foundation, Alfred P. Sloan Foundation, ISI Foundation, Paul G. Allen Family Foundation, Army Research Office (Bassett-W911NF-14-1-0679), Office of Naval Research, National Institute of Mental Health (2-R01-DC-009209-11, R01 MH-112847, R01 MH-107235, R21 MH-106799 and R01 MH-113550), National Institute of Child Health and Human Development (1R01HD086888-01), National Institute of Neurological Disorders and Stroke (R01 NS099348) and National Science Foundation (BCS-1441502, BCS-1631550 and CNS-1626008). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.E.K., E.A.K., J.M.V. and D.S.B. conceived the project and planned the experiments and analyses. A.E.K. performed the experiments and analyses. A.E.K., E.A.K. and D.S.B. wrote the manuscript and Supplementary Information. E.A.K., J.M.V. and D.S.B. edited the manuscript and Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–10, Supplementary Tables 1–6
Rights and permissions
About this article
Cite this article
Kahn, A.E., Karuza, E.A., Vettel, J.M. et al. Network constraints on learnability of probabilistic motor sequences. Nat Hum Behav 2, 936–947 (2018). https://doi.org/10.1038/s41562-018-0463-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0463-8
This article is cited by
-
Methodological considerations for behavioral studies relying on response time outcomes through online crowdsourcing platforms
Scientific Reports (2024)
-
Response times are affected by mispredictions in a stochastic game
Scientific Reports (2024)
-
Sleep targets highly connected global and local nodes to aid consolidation of learned graph networks
Scientific Reports (2022)
-
Predicting memory from the network structure of naturalistic events
Nature Communications (2022)
-
Abstract representations of events arise from mental errors in learning and memory
Nature Communications (2020)