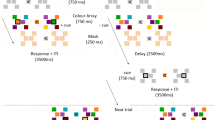
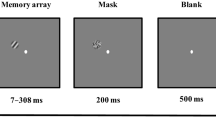
Abstract
After encoding, memory traces are fragile and easily disrupted by new learning until they are stabilized through a process termed consolidation1,2. However, several studies have suggested that consolidation does not make memory traces permanently stable. The results of these studies support the theory that the retrieval of previously consolidated memory, termed reactivation, renders the memory traces labile again and subject to disruption by new learning unless they go through a further consolidation process, termed reconsolidation3,4,5,6,7,8. However, it remains controversial whether reactivation and reconsolidation occur at a human behavioural level9,10,11 and whether consolidation and reconsolidation have common mechanisms12,13. Here, we found that reconsolidation does occur after reactivation in visual perceptual learning14,15,16,17,18,19,20,21,22,23,24,25, a type of skill learning, in humans. Moreover, changes in behavioural performance, as well as in concentrations in the excitatory neurotransmitter glutamate and in the inhibitory neurotransmitter GABA (γ-aminobutyric acid), as measured by magnetic resonance spectroscopy, in early visual areas exhibit similar time courses during consolidation and reconsolidation. These results indicate that reconsolidation after reactivation and consolidation in humans share common behavioural and neurochemical mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alvarez, P. & Squire, L. R. Memory consolidation and the medial temporal lobe: a simple network model. Proc. Natl Acad. Sci. USA 91, 7041–7045 (1994).
Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004).
Dayan, E., Laor-Maayany, R. & Censor, N. Reward disrupts reactivated human skill memory. Sci. Rep. 6, 28270 (2016).
Monfils, M. H., Cowansage, K. K., Klann, E. & LeDoux, J. E. Extinction–reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955 (2009).
Bjorkstrand, J. et al. Disrupting reconsolidation attenuates long-term fear memory in the human amygdala and facilitates approach behavior. Curr. Biol. 26, 2690–2695 (2016).
Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000).
Walker, M. P., Brakefield, T., Hobson, J. A. & Stickgold, R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620 (2003).
Robertson, E. M. New insights in human memory interference and consolidation. Curr. Biol. 22, R66–R71 (2012).
Wood, N. E. et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 225, 31–39 (2015).
Bos, M. G., Beckers, T. & Kindt, M. Noradrenergic blockade of memory reconsolidation: a failure to reduce conditioned fear responding. Front. Behav. Neurosci. 8, 412 (2014).
Hardwicke, T. E., Taqi, M. & Shanks, D. R. Postretrieval new learning does not reliably induce human memory updating via reconsolidation. Proc. Natl Acad. Sci. USA 113, 5206–5211 (2016).
Lee, J. L., Everitt, B. J. & Thomas, K. L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843 (2004).
Debiec, J., Doyere, V., Nader, K. & Ledoux, J. E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl Acad. Sci. USA 103, 3428–3433 (2006).
Karni, A. & Sagi, D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc. Natl Acad. Sci. USA 88, 4966–4970 (1991).
Ahissar, M. & Hochstein, S. Task difficulty and the specificity of perceptual learning. Nature 387, 401–406 (1997).
Dosher, B. A. & Lu, Z. L. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc. Natl Acad. Sci. USA 95, 13988–13993 (1998).
Yu, Q., Zhang, P., Qiu, J. & Fang, F. Perceptual learning of contrast detection in the human lateral geniculate nucleus. Curr. Biol. 26, 3176–3182 (2016).
Amar-Halpert, R., Laor-Maayany, R., Nemni, S., Rosenblatt, J. & Censor, N. Memory reactivation improves visual perception. Nat. Neurosci. 20, 1325–1328 2017).
Xiao, L. Q. et al. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr. Biol. 18, 1922–1926 2008).
Watanabe, T. et al. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nat. Neurosci. 5, 1003–1009 2002).
Watanabe, T., Nanez, J. E. & Sasaki, Y. Perceptual learning without perception. Nature 413, 844–848 (2001).
Seitz, A. R. & Watanabe, T. Psychophysics: is subliminal learning really passive? Nature 422, 36 (2003).
Yotsumoto, Y., Watanabe, T. & Sasaki, Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57, 827–833 (2008).
Shibata, K. et al. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nat. Neurosci. 20, 470–475 (2017).
Shibata, K., Watanabe, T., Sasaki, Y. & Kawato, M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334, 1413–1415 (2011).
Nikolova, S., Stark, S. M. & Stark, C. E. 3T hippocampal glutamate–glutamine complex reflects verbal memory decline in aging. Neurobiol. Aging 54, 103–111 (2017).
Kim, S., Stephenson, M. C., Morris, P. G. & Jackson, S. R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. NeuroImage 99, 237–243 (2014).
Stagg, C. J., Bachtiar, V. & Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 21, 480–484 (2011).
Hensch, T. K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Stagg, C. J. Magnetic resonance spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. NeuroImage 86, 19–27 (2014).
Okubo, Y. et al. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl Acad. Sci. USA 107, 6526–6531 (2010).
Myers, J. F., Evans, C. J., Kalk, N. J., Edden, R. A. & Lingford-Hughes, A. R. Measurement of GABA using J-difference edited 1H-MRS following modulation of synaptic GABA concentration with tiagabine. Synapse 68, 355–362 (2014).
Watanabe, T. & Sasaki, Y. Perceptual learning: toward a comprehensive theory. Annu. Rev. Psychol. 66, 197–221 (2015).
Sasaki, Y., Nanez, J. E. & Watanabe, T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 11, 53–60 (2010).
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Hu, Y., Chen, X., Gu, H. & Yang, Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 33, 18566–18573 (2013).
Mescher, M., Merkle, H., Kirsch, J., Garwood, M. & Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272 (1998).
Rothman, D. L., Behar, K. L., Hetherington, H. P. & Shulman, R. G. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proc. Natl Acad. Sci. USA 81, 6330–6334 (1984).
Robertson, C. E., Ratai, E. M. & Kanwisher, N. Reduced GABAergic action in the autistic brain. Curr. Biol. 26, 80–85 (2016).
Hancu, I. Optimized glutamate detection at 3T. J. Magn. Reson. Imaging 30, 1155–1162 (2009).
Mullins, P. G., Chen, H., Xu, J., Caprihan, A. & Gasparovic, C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn. Reson. Med. 60, 964–969 (2008).
Tkac, I., Starcuk, Z., Choi, I. Y. & Gruetter, R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 41, 649–656 (1999).
Provencher, S. W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 (1993).
Provencher, S. W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14, 260–264 (2001).
Acknowledgements
This work was supported by the NIH (R01EY019466), the NSF (BCS 1539717) and the JSPS KAKENHI grant number 17H04789. The funding agencies had no role in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
This work was conceived by J.W.B., S.M.F., T.W. and Y.S. J.W.B., K.S., S.M.F. and E.G.W. collected and analysed the data. J.W.B., K.S., S.M.F., E.G.W., M.W.G., T.W. and Y.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Supplementary Figures 1–2
Rights and permissions
About this article
Cite this article
Bang, J.W., Shibata, K., Frank, S.M. et al. Consolidation and reconsolidation share behavioural and neurochemical mechanisms. Nat Hum Behav 2, 507–513 (2018). https://doi.org/10.1038/s41562-018-0366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0366-8
This article is cited by
-
Early excitatory-inhibitory cortical modifications following skill learning are associated with motor memory consolidation and plasticity overnight
Nature Communications (2024)
-
Long term structural and functional neural changes following a single infusion of Ketamine in PTSD
Neuropsychopharmacology (2023)
-
The phase of plasticity-induced neurochemical changes of high-frequency repetitive transcranial magnetic stimulation are different from visual perceptual learning
Scientific Reports (2023)
-
Reactivation-induced motor skill modulation does not operate at a rapid micro-timescale level
Scientific Reports (2023)
-
Astrocyte-derived lactate/NADH alters methamphetamine-induced memory consolidation and retrieval by regulating neuronal synaptic plasticity in the dorsal hippocampus
Brain Structure and Function (2022)