Abstract
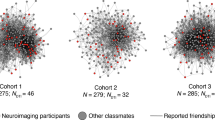
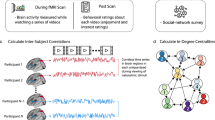
Unlike many species that enact social behaviour in loose aggregations (such as swarms or herds), humans form groups comprising many long-term, intense, non-reproductive bonds with non-kin1. The cognitive demands of navigating such groups are thought to have significantly influenced human brain evolution2. Yet little is known about how and to what extent the human brain encodes the structure of the social networks in which it is embedded. We characterized the social network of an academic cohort (N = 275); a subset (N = 21) completed a functional magnetic resonance imaging (fMRI) study involving viewing individuals who varied in terms of ‘degrees of separation’ from themselves (social distance), the extent to which they were well-connected to well-connected others (eigenvector centrality) and the extent to which they connected otherwise unconnected individuals (brokerage). Understanding these characteristics of social network position requires tracking direct relationships, bonds between third parties and the broader network topology. Pairing network data with multi-voxel pattern analysis, we show that information about social network position is accurately perceived and spontaneously activated when encountering familiar individuals. These findings elucidate how the human brain encodes the structure of its social world and underscore the importance of integrating an understanding of social networks into the study of social perception.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shultz, S. & Dunbar, R. I. M. Bondedness and sociality. Behaviour 147, 775–803 (2010).
Dunbar, R. I. M. & Shultz, S. Evolution in the social brain. Science 317, 1344–1347 (2007).
Ellwardt, L., Labianca, G. & Wittek, R. Who are the objects of positive and negative gossip at work? Soc. Networks 34, 193–205 (2012).
Burt, R. S., Kilduff, M. & Tasselli, S. Social network analysis: foundations and frontiers on advantage. Annu. Rev. Psychol. 64, 527–547 (2013).
Burt, R. S. & Knez, M. Kinds of third-party effects on trust. Ration. Soc. 7, 255–292 (1995).
Brent, L. J. N. Friends of friends: are indirect connections in social networks important to animal behaviour? Anim. Behav. 103, 211–222 (2015).
Uleman, J. S ., Newman, L. S. & Moskowitz, G. B. in Advances in Experimental Social Psychology Vol. 28 (ed. Zanna, P. ) 211–279 (Academic, 1996).
Todorov, A., Gobbini, M. I., Evans, K. K. & Haxby, J. V. Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia 45, 163–173 (2007).
Bonacich, P. Power and centrality: a family of measures. Am. J. Sociol. 92, 1170–1182 (1987).
Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis: connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008).
Chikazoe, J., Lee, D. H., Kriegeskorte, N. & Anderson, A. K. Population coding of affect across stimuli, modalities and individuals. Nat. Neurosci. 17, 1114–1122 (2014).
Tennie, C., Frith, U. & Frith, C. D. Reputation management in the age of the world-wide web. Trends Cogn. Sci. 14, 482–488 (2010).
Krienen, F. M., Tu, P.-C. & Buckner, R. L. Clan mentality: evidence that the medial prefrontal cortex responds to close others. J. Neurosci. 30, 13906–13915 (2010).
Parkinson, C., Liu, S. & Wheatley, T. A common cortical metric for spatial, temporal, and social distance. J. Neurosci. 34, 1979–1987 (2014).
Gauthier, B. & van Wassenhove, V. Time is not space: core computations and domain-specific networks for mental travels. J. Neurosci. 36, 11891–11903 (2016).
Yamazaki, Y., Hashimoto, T. & Iriki, A. The posterior parietal cortex and non-spatial cognition. F1000 Biol. Rep. 1, 74 (2009).
Parkinson, C. & Wheatley, T. Old cortex, new contexts: re-purposing spatial perception for social cognition. Front. Hum. Neurosci. 7, 645 (2013).
Parkinson, C. & Wheatley, T. The repurposed social brain. Trends Cogn. Sci. 19, 133–141 (2015).
Tavares, R. M. et al. A map for social navigation in the human brain. Neuron 87, 231–243 (2015).
Fowler, J. H., Dawes, C. T. & Christakis, N. A. Model of genetic variation in human social networks. Proc. Natl Acad. Sci. USA 106, 1720–1724 (2009).
Burt, R. S. Network-related personality and the agency question: multirole evidence from a virtual world. Am. J. Sociol. 118, 543–591 (2012).
Wagner, D. D., Haxby, J. V. & Heatherton, T. F. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdiscip. Rev. Cogn. Sci. 3, 451–470 (2012).
Hassabis, D. et al. Imagine all the people: how the brain creates and uses personality models to predict behavior. Cereb. Cortex 24, 1979–1987 (2014).
Kriegeskorte, N., Formisano, E., Sorger, B. & Goebel, R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc. Natl Acad. Sci. USA 104, 20600–20605 (2007).
Nestor, A., Plaut, D. C. & Behrmann, M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc. Natl Acad. Sci. USA 108, 9998–10003 (2011).
Feiler, D. C. & Kleinbaum, A. M. Popularity, similarity, and the network extraversion bias. Psychol. Sci. 26, 593–603 (2015).
Zerubavel, N., Bearman, P. S., Weber, J. & Ochsner, K. N. Neural mechanisms tracking popularity in real-world social networks. Proc. Natl Acad. Sci. USA 112, 15072–15077 (2015).
Jones, B. C. et al. Facial cues of dominance modulate the short-term gaze-cuing effect in human observers. Proc. R. Soc. B 277, 617–624 (2010).
Dalmaso, M., Pavan, G., Castelli, L. & Galfano, G. Social status gates social attention in humans. Biol. Lett. 8, 450–452 (2012).
Klein, J. T., Shepherd, S. V. & Platt, M. L. Social attention and the brain. Curr. Biol. 19, R958–R962 (2009).
Shepherd, S. V., Deaner, R. O. & Platt, M. L. Social status gates social attention in monkeys. Curr. Biol. 16, R119–R120 (2006).
Marsh, A. A., Blair, K. S., Jones, M. M., Soliman, N. & Blair, R. J. R. Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J. Cogn. Neurosci. 21, 713–724 (2009).
Cloutier, J. & Gyurovski, I. Ventral medial prefrontal cortex and person evaluation: forming impressions of others varying in financial and moral status. Neuroimage 100, 535–543 (2014).
Karafin, M. S., Tranel, D. & Adolphs, R. Dominance attributions following damage to the ventromedial prefrontal cortex. J. Cogn. Neurosci. 16, 1796–1804 (2004).
Pinker, S. Decline of violence: taming the devil within us. Nature 478, 309–311 (2011).
Grossman, E. D., Battelli, L. & Pascual-Leone, A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res. 45, 2847–2853 (2005).
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M. & Fried, I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 20, 750–756 (2010).
Wheatley, T., Milleville, S. C. & Martin, A. Understanding animate agents: distinct roles for the social network and mirror system. Psychol. Sci. 18, 469–474 (2007).
Burt, R. S. Structural Holes: The Social Structure of Competition (Harvard Univ. Press, 1992).
Kleinbaum, A. M., Jordan, A. H. & Audia, P. G. An altercentric perspective on the origins of brokerage in social networks: how perceived empathy moderates the self-monitoring effect. Organ. Sci. 26, 1226–1242 (2015).
R Core Development Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012).
Csardi, G. & Nepusz, T. The igraph software package for complex network research. InterJ. Complex Syst. 1695 (2006).
Connolly, A. C. et al. The representation of biological classes in the human brain. J. Neurosci. 32, 2608–2618 (2012).
Said, C. P., Moore, C. D., Engell, A. D. & Haxby, J. V. Distributed representations of dynamic facial expressions in the superior temporal sulcus. J. Vis. 10, 1–12 (2010).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Satterthhwaite, F. E. An approximate distribution of estimates of variance components. Biometrics 2, 110–114 (1946).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest: tests in linear mixed effects models. R Package v 2.0-33 (CRAN, 2016).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Hanke, M. et al. PyMVPA: a unifying approach to the analysis of neuroscientific data. Front. Neuroinform. 3, 3 (2009).
Oliphant, T. E. SciPy: open source scientific tools for Python. Comput. Sci. Eng. 9, 10–20 (2007).
Talairach, J. & Tournoux, P. Co-Planar Stereotaxis Atlas of the Human Brain (Thieme Medical, 1988).
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397 (2014).
Jenkinson, M ., Beckmann, C. F ., Behrens, T. E. J ., Woolrich, M. W. & Smith, S. M. FSL. NeuroImage 62, 782–790 (2012).
Acknowledgements
This work was supported by a graduate fellowship from the Neukom Institute for Computational Science and a Dartmouth Graduate Alumni Research Award to C.P. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors thank W. Haslett for assistance with the optical flow analysis.
Author information
Authors and Affiliations
Contributions
C.P., A.M.K. and T.W. conceived and designed the study. C.P. and A.M.K. collected the data. C.P. analysed the data. C.P., A.M.K. and T.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–4, Supplementary Tables 1–3, Supplementary Methods, Supplementary References. (PDF 586 kb)
Rights and permissions
About this article
Cite this article
Parkinson, C., Kleinbaum, A. & Wheatley, T. Spontaneous neural encoding of social network position. Nat Hum Behav 1, 0072 (2017). https://doi.org/10.1038/s41562-017-0072
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-017-0072
This article is cited by
-
Strengths of social ties modulate brain computations for third-party punishment
Scientific Reports (2023)
-
Neural signatures of social inferences predict the number of real-life social contacts and autism severity
Nature Communications (2023)
-
Neurocomputational mechanism of real-time distributed learning on social networks
Nature Neuroscience (2023)
-
Overlap between mental representations of nationalities modulates perceptual matching
Current Psychology (2023)
-
In-degree centrality in a social network is linked to coordinated neural activity
Nature Communications (2022)