Abstract
Nickel is a biologically essential element for marine life, with the potential to influence diverse processes, including methanogenesis, nitrogen uptake and coral health, in both modern and past oceans. However, an incomplete view of oceanic Ni cycling has stymied understanding of how Ni may impact marine life in these modern and ancient oceans. Here we combine data-constrained global biogeochemical circulation modelling with culture experiments and find that Ni in oligotrophic gyres is both chemically and biologically labile and only minimally incorporated into diatom frustules. We then develop a framework for understanding oceanic Ni distributions, and in particular the two dominant features of the global marine Ni distribution: the deep concentration maximum and the residual pool of approximately 2 nM Ni in subtropical gyres. We suggest that slow depletion of Ni relative to macronutrients in upwelling regions can explain the residual Ni pool, and reversible scavenging or slower regeneration of Ni compared with macronutrients contributes to the distinct Ni vertical distribution. The strength of these controls may have varied in the past ocean, impacting Ni bioavailability and setting a fine balance between Ni feast and famine for phytoplankton, with implications for both ocean chemistry and climate state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data from the GEOTRACES 2017 IDP are available at https://www.bodc.ac.uk/geotraces/data/idp2017/. Data from the Tara Pacific expedition are available at https://doi.org/10.1594/PANGAEA.875582. Data from the US GEOTRACES GP15 transect are available at https://github.com/MTEL-USC/nickel-model.
Code availability
Model code for this work is available at https://github.com/MTEL-USC/nickel-model and can be run within the AWESOME OCIM modelling environment available at https://github.com/profseth/awesomeOCIM.
References
Alfano, M. & Cavazza, C. Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci. 29, 1071–1089 (2020).
Glass, J. B. & Dupont, C. L. in The Biological Chemistry of Nickel (eds Zamble, D., Rowinska-Zyrek, M. & Kozlowski, H.) 12–26 (The Royal Society of Chemistry, 2017); https://doi.org/10.1039/9781788010580-00012
Ho, T.-Y. Nickel limitation of nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 58, 112–120 (2013).
Tuo, S., Rodriguez, I. B. & Ho, T.-Y. H2 accumulation and N2 fixation variation by Ni limitation in Cyanothece. Limnol. Oceanogr. 65, 377–386 (2020).
Dupont, C. L., Buck, K. N., Palenik, B. & Barbeau, K. Nickel utilization in phytoplankton assemblages from contrasting oceanic regimes. Deep Sea Res. 1 57, 553–566 (2010).
Biscéré, T. et al. Enhancement of coral calcification via the interplay of nickel and urease. Aquat. Toxicol. 200, 247–256 (2018).
Konhauser, K. O. et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750–753 (2009).
Wang, S.-J., Rudnick, R. L., Gaschnig, R. M., Wang, H. & Wasylenki, L. E. Methanogenesis sustained by sulfide weathering during the Great Oxidation Event. Nat. Geosci. 12, 296–300 (2019).
Zhao, Z. et al. Active methanogenesis during the melting of Marinoan snowball Earth. Nat. Commun. 12, 955 (2021).
Sunda, W. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 3, 204 (2012).
Sarmiento, J. L., Gruber, N., Brzezinski, M. A. & Dunne, J. P. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427, 56–60 (2004).
Weber, T., John, S., Tagliabue, A. & DeVries, T. Biological uptake and reversible scavenging of zinc in the global ocean. Science 361, 72–76 (2018).
Price, N. M. & Morel, F. M. M. Colimitation of phytoplankton growth by nickel and nitrogen. Limnol. Oceanogr. 36, 1071–1077 (1991).
Mackey, D. J., O’Sullivan, J. E., Watson, R. J. & Dal Pont, G. Trace metals in the western Pacific: temporal and spatial variability in the concentrations of Cd, Cu, Mn and Ni. Deep Sea Res. 1 49, 2241–2259 (2002).
Wen, L.-S., Jiann, K.-T. & Santschi, P. H. Physicochemical speciation of bioactive trace metals (Cd, Cu, Fe, Ni) in the oligotrophic South China Sea. Mar. Chem. 101, 104–129 (2006).
Archer, C., Vance, D., Milne, A. & Lohan, M. C. The oceanic biogeochemistry of nickel and its isotopes: new data from the South Atlantic and the Southern Ocean biogeochemical divide. Earth Planet. Sci. Lett. 535, 116118 (2020).
Lemaitre, N., Du, J., de Souza, G. F., Archer, C. & Vance, D. The essential bioactive role of nickel in the oceans: evidence from nickel isotopes. Earth Planet. Sci. Lett. 584, 117513 (2022).
Achterberg, E. P. & Van Den Berg, C. M. G. Chemical speciation of chromium and nickel in the western Mediterranean. Deep Sea Res. 2 44, 693–720 (1997).
Twining, B. S., Baines, S. B., Vogt, S. & Nelson, D. M. Role of diatoms in nickel biogeochemistry in the ocean. Glob. Biogeochem. Cycles 26 (2012).
Middag, R., de Baar, H. J. W., Bruland, K. W. & van Heuven, S. M. A. C. The distribution of nickel in the west-Atlantic Ocean, its relationship with phosphate and a comparison to cadmium and zinc. Front. Mar. Sci. 7, 105 (2020).
Twining, B. S. et al. Metal quotas of plankton in the equatorial Pacific Ocean. Deep Sea Res. 2 58, 325–341 (2011).
Saito, M. A., Moffett, J. W. & DiTullio, G. R. Cobalt and nickel in the Peru upwelling region: a major flux of labile cobalt utilized as a micronutrient. Glob. Biogeochem. Cycles 18 (2004).
John, S. G. et al. AWESOME OCIM: a simple, flexible, and powerful tool for modeling elemental cycling in the oceans. Chem. Geol. 533, 119403 (2020).
DeVries, T., Holzer, M. & Primeau, F. Recent increase in oceanic carbon uptake driven by weaker upper-ocean overturning. Nature 542, 215 (2017).
Dupont, C. L., Barbeau, K. & Palenik, B. Ni uptake and limitation in marine Synechococcus strains. Appl. Environ. Microbiol. 74, 23–31 (2008).
Twining, B. S. et al. Differential remineralization of major and trace elements in sinking diatoms. Limnol. Oceanogr. 59, 689–704 (2014).
Zheng, L., Minami, T., Takano, S., Ho, T.-Y. & Sohrin, Y. Sectional distribution patterns of Cd, Ni, Zn, and Cu in the North Pacific Ocean: relationships to nutrients and importance of scavenging. Glob. Biogeochem. Cycles 35, e2020GB006558 (2021).
Le Gland, G., Aumont, O. & Mémery, L. An estimate of thorium 234 partition coefficients through global inverse modeling. J. Geophys. Res. Ocean. 124, 3575–3606 (2019).
Buesseler, K. O. et al. Revisiting carbon flux through the ocean’s twilight zone. Science 316, 567–570 (2007).
Weber, T., Cram, J. A., Leung, S. W., DeVries, T. & Deutsch, C. Deep ocean nutrients imply large latitudinal variation in particle transfer efficiency. Proc. Natl Acad. Sci. USA 113, 8606 LP–8608611 (2016).
Klaas, C. & Archer, D. E. Association of sinking organic matter with various types of mineral ballast in the deep sea: implications for the rain ratio. Glob. Biogeochem. Cycles 16, 14–63 (2002).
Berelson, W. M. The flux of particulate organic carbon into the ocean interior: a comparison of four US JGOFS regional studies. Oceanography 14, 59–67 (2001).
Kooistra, W. H. C. F., Gersonde, R., Medlin, L. K. & Mann, D. G. in Evolution of Primary Produces in the Sea (eds Falkowski, P. G. & Knoll, A. H.) 207–249 (Academic Press, 2007); https://doi.org/10.1016/B978-012370518-1/50012-6
Medlin, L. K., Kooistra, W. C. H. F. & Schmid, A.-M. M. in The Origin and Early Evolution of the Diatoms: Fossil, Molecular and Biogeographical Approaches (eds Witkowski, A. & Siemińska, J.) 13–35 (Polish Academy of Sciences, 2000).
Rodriguez, I. B. & Ho, T.-Y. Diel nitrogen fixation pattern of Trichodesmium: the interactive control of light and Ni. Sci. Rep. 4, 4445 (2014).
Algeo, T. J., Meyers, P. A., Robinson, R. S., Rowe, H. & Jiang, G. Q. Icehouse–greenhouse variations in marine denitrification. Biogeosciences 11, 1273–1295 (2014).
Little, S. H. et al. Towards balancing the oceanic Ni budget. Earth Planet. Sci. Lett. 547, 116461 (2020).
Ciscato, E. R., Bontognali, T. R. R. & Vance, D. Nickel and its isotopes in organic-rich sediments: implications for oceanic budgets and a potential record of ancient seawater. Earth Planet. Sci. Lett. 494, 239–250 (2018).
Roy, S. Sedimentary manganese metallogenesis in response to the evolution of the Earth system. Earth Sci. Rev. 77, 273–305 (2006).
Donat, J. R., Lao, K. A. & Bruland, K. W. Speciation of dissolved copper and nickel in South San Francisco Bay: a multi-method approach. Anal. Chim. Acta 284, 547–571 (1994).
Hawco, N. J. et al. Metal isotope signatures from lava–seawater interaction during the 2018 eruption of Kīlauea. Geochim. Cosmochim. Acta 282, 340–356 (2020).
Saad, E. M. et al. Effect of cleaning methods on the dissolution of diatom frustules. Mar. Chem. 224, 103826 (2020).
Andersen, M. B. et al. The Zn abundance and isotopic composition of diatom frustules, a proxy for Zn availability in ocean surface seawater. Earth Planet. Sci. Lett. 301, 137–145 (2011).
Ellwood, M. J. & Hunter, K. A. The incorporation of zinc and iron into the frustule of the marine diatom Thalassiosira pseudonana. Limnol. Oceanogr. 45, 1517–1524 (2000).
Sunda, W. G., Price, N. M. & Morel, F. M. M. Trace metal ion buffers and their use in culture studies. Algal Cult. Tech. 4, 35–63 (2005).
Tovar-Sanchez, A. et al. A trace metal clean reagent to remove surface-bound iron from marine phytoplankton. Mar. Chem. 82, 91–99 (2003).
Capone, D. G. & Hutchins, D. A. Microbial biogeochemistry of coastal upwelling regimes in a changing ocean. Nat. Geosci. 6, 711–717 (2013).
Cutter, G. et al. Sampling and Sample-Handling Protocols for GEOTRACES Cruises (GEOTRACES International Project Office, 2017); https://doi.org/10.25607/OBP-2
Schlitzer, R. et al. The GEOTRACES Intermediate Data Product 2017. Chem. Geol. 493, 210–223 (2018).
Gorsky, G. et al. Expanding Tara oceans protocols for underway, ecosystemic sampling of the ocean–atmosphere interface during Tara Pacific expedition (2016–2018). Front. Mar. Sci. 6, 750 (2019).
Jensen, L. T., Wyatt, N. J., Landing, W. M. & Fitzsimmons, J. N. Assessment of the stability, sorption, and exchangeability of marine dissolved and colloidal metals. Mar. Chem. 220, 103754 (2020).
DeVries, T. The oceanic anthropogenic CO2 sink: storage, air–sea fluxes, and transports over the industrial era. Glob. Biogeochem. Cycles 28, 631–647 (2014).
Holzer, M., Primeau, F. W., DeVries, T. & Matear, R. The Southern Ocean silicon trap: data-constrained estimates of regenerated silicic acid, trapping efficiencies, and global transport paths. J. Geophys. Res. Oceans 119, 313–331 (2014).
van Hulten, M., Dutay, J.-C. & Roy-Barman, M. A global scavenging and circulation ocean model of thorium-230 and protactinium-231 with improved particle dynamics (NEMO–ProThorP~0.1). Geosci. Model Dev. 11, 3537–3556 (2018).
van Hulten, M. et al. Manganese in the west Atlantic Ocean in the context of the first global ocean circulation model of manganese. Biogeosciences 14, 1123–1152 (2017).
Westall, J. C. et al. MINEQL: A Computer Program for the Calculation of Chemical Equilibrium Composition of Aqueous Systems (Water Quality Laboratory, Ralph M. Parsons Laboratory for Water Resources and Environmental Engineering, and Department of Civil Engineering, Massachusetts Institute of Technology, 1976).
Biscéré, T. et al. Nickel and ocean warming affect scleractinian coral growth. Mar. Pollut. Bull. 120, 250–258 (2017).
Oliveira, L. & Antia, N. J. Evidence of nickel ion requirement for autotrophic growth of a marine diatom with urea serving as nitrogen source. Br. Phycol. J. 19, 125–134 (1984).
Oliveira, L. & Antia, N. J. Nickel ion requirements for autotrophic growth of several marine microalgae with urea serving as nitrogen source. Can. J. Fish. Aquat. Sci. 43, 2427–2433 (1986).
Ho, T.-Y., Chu, T.-H. & Hu, C.-L. Interrelated influence of light and Ni on Trichodesmium growth. Front. Microbiol. 4, 139 (2013).
Nuester, J., Vogt, S., Newville, M., Kustka, A. & Twining, B. The unique biogeochemical signature of the marine diazotroph Trichodesmium. Front. Microbiol. 3, 150 (2012).
Twining, B. S., Nuñez-Milland, D., Vogt, S., Johnson, R. S. & Sedwick, P. N. Variations in Synechococcus cell quotas of phosphorus, sulfur, manganese, iron, nickel, and zinc within mesoscale eddies in the Sargasso Sea. Limnol. Oceanogr. 55, 492–506 (2010).
Acknowledgements
Thanks to the many scientists who contributed data to the International GEOTRACES 2017 International Data Product, including the captains and crew of research vessels, the technicians who collected samples at sea and the analysts. Funding was provided by the Simons Foundation (award # 426570SP to S.G.J.), the Australian Research Council (award # DP210101650 to M.H.) and the National Science Foundation (award #s 1736896, 1737136, 1737167, 1851222 and 1746932 to S.G.J., T.M.C., J.N.F., D.A.H. and N.T.L., respectively).
Author information
Authors and Affiliations
Contributions
Phytoplankton culturing and analysis of culture samples was done by R.L.K., X.B., S.-C.Y., E.A.S., F.F., M.I.S. and D.A.H. Analyses of natural materials and seawater samples were completed by S.-C.Y., X.B., N.T.L., J.N.F. and T.M.C. Chemical experiments and analysis were performed by S.G.J. Modelling was undertaken by S.G.J. with the assistance of H.L., B.P., M.H. and L.W. The manuscript was written by S.G.J. with advice and input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Benjamin Twining and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rebecca Neely, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Base model output.
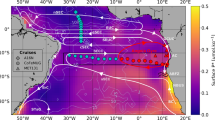
Patterns in vertical and horizontal distribution of simulated Ni for a model including only biological uptake and remineralization of Ni in soft-tissue. a) Comparison between observations (colored circles) and optimized model output (background color) are shown for the surface ocean, with white lines delineating the boundaries of the oligotrophic gyre at 0.2 μM PO42-, and black lines showing the location of depth transect data. b) Comparison between observations and optimized model output are shown for depth transects in the Atlantic and Pacific Ocean, which include GEOTRACES transects GA02 and GP15, respectively. The Ni mean depth reported above this panel refers to the average depth of model-predicted Ni in the global ocean, which can be compared to mean depths of 2174 m and 2533 m for P and Si, respectively, based on World Ocean Atlas 2009 data. c) The global fit between model and observed Ni, with the colorscale reflecting the relative data density as a percentage compared to maximum data density. d) Horizontal patterns in global depth integrated scavenging flux of Ni (which has no value for this model because no scavenging process was included), presented as a percentage of the maximum scavenging intensity. e) vertical patterns in horizontally integrated Ni scavenging flux (which has no value for this model because no scavenging process was included), presented as a percentage of the maximum scavenging intensity. Additional information about optimized model parameters and model performance metrics are presented in Table ED1.
Extended Data Fig. 2 Model output with Ni in frustules.
Patterns in vertical and horizontal distribution of simulated Ni for a model including biological uptake and remineralization of Ni in soft-tissue, and the biological uptake and remineralization of Ni due to incorporation in diatom silicate frustules. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 4 Model output with reversible scavenging like Th.
Patterns in vertical and horizontal distribution of simulated Ni can be evaluated for models with various parameterizations of reversible scavenging, here showing a model with reversible scavenging taking the same patterns as Th scavenging from Hulten et al.54. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 5 Model output with reversible scavenging like Pa.
Patterns in vertical and horizontal distribution of simulated Ni can be evaluated for models with various parameterizations of reversible scavenging, here showing a model with reversible scavenging taking the same patterns as Pa scavenging from Hulten et al.54. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 6 Model output with reversible scavenging onto Mn oxides.
Patterns in vertical and horizontal distribution of simulated Ni can be evaluated for models with various parameterizations of reversible scavenging, here showing a model with reversible scavenging onto particulate Mn oxides, based on a Mn model from van Hulten et al.55. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 7 Model output with reversible scavenging onto POC, variable b.
Patterns in vertical and horizontal distribution of simulated Ni can be evaluated for models with various parameterizations of reversible scavenging, here showing a model with reversible scavenging onto POC based on Weber et al.12, with the vertical distributions of scavenging sites on POC determined from an optimized power-law equation. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 8 Model output with deeper organic Ni remineralization.
Patterns in vertical and horizontal distribution of simulated Ni, here showing a model where Ni is allowed to remineralize according to a ‘Martin curve’ power law, except that the b exponent is optimizable for Ni instead of being tied to the remineralization of P. Panels are the same as for Extended Data Fig. 1.
Extended Data Fig. 9 Diatoms collected from the North Pacific.
Light microscopy micrographs showing persistence of intact and undamaged biogenic silica shells after removal of cellular organic material using HNO3. Shown are HNO3 cleaned silica frustules of a) the centric diatom Triceratium (100X magnification), b) the pennate diatom Pseudo-nitzschia (100X magnification), and c) the centric diatom Coscinodiscus (400X magnification). Even delicate shells of d) the silicoflagellate Dictyocha (100X magnification) came through the HNO3 digestion procedure intact, as did similarly fragile silica shells of radiolarians (not shown). All cells shown were collected on a 53 µm filter.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
John, S.G., Kelly, R.L., Bian, X. et al. The biogeochemical balance of oceanic nickel cycling. Nat. Geosci. 15, 906–912 (2022). https://doi.org/10.1038/s41561-022-01045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-01045-7