Abstract
Secondary organic aerosol contributes a significant fraction to aerosol mass and toxicity. Low-volatility organic vapours are critical intermediates connecting the oxidation of volatile organic compounds to secondary organic aerosol formation. However, the direct measurement of intermediate vapours poses a great challenge. Here we present coordinated measurements of oxygenated organic molecules in the three most urbanized regions of China and determine their likely precursors, enabling us to connect secondary organic aerosol formation to various volatile organic compounds. We show that the oxidation of anthropogenic volatile organic compounds dominates oxygenated organic molecule formation, with an approximately 40% contribution from aromatics and a 40% contribution from aliphatic hydrocarbons (predominantly alkanes), a previously under-accounted class of volatile organic compounds. The irreversible condensation of these anthropogenic oxygenated organic molecules increases significantly in highly polluted conditions, accounting for a major fraction of the production of secondary organic aerosol. We find that the distribution of oxygenated organic molecules and their formation pathways are largely the same across the urbanized regions. This suggests that uniform mitigation strategies could be effective in solving air pollution issues across these highly populated city clusters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The observation data that support the main findings of this study are available at figshare (https://doi.org/10.6084/m9.figshare.14526801.v1). The aerosol optical depth data used in this work are archived at https://atmosphere-imager.gsfc.nasa.gov/products/monthly. Source data are provided with this paper.
Code availability
Data processing techniques are available on request from the corresponding author.
References
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013).
Heal, M. R., Kumar, P. & Harrison, R. M. Particles, air quality, policy and health. Chem. Soc. Rev. 41, 6606–6630 (2012).
Jimenez, J. L. et al. Evolution of organic aerosols in the atmosphere. Science 326, 1525–1529 (2009).
Huang, R. J. et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 514, 218–222 (2014).
Zhou, J. et al. Predominance of secondary organic aerosol to particle-bound reactive oxygen species activity in fine ambient aerosol. Atmos. Chem. Phys. 19, 14703–14720 (2019).
Laskin, A., Laskin, J. & Nizkorodov, S. Mass spectrometric approaches for chemical characterisation of atmospheric aerosols: critical review of the most recent advances. Environ. Chem. 9, 163–189 (2012).
Nozière, B. et al. The molecular identification of organic compounds in the atmosphere: state of the art and challenges. Chem. Rev. 115, 3919–3983 (2015).
Isaacman-VanWertz, G. et al. Chemical evolution of atmospheric organic carbon over multiple generations of oxidation. Nat. Chem. 10, 462–468 (2018).
Yan, C. et al. Source characterization of highly oxidized multifunctional compounds in a boreal forest environment using positive matrix factorization. Atmos. Chem. Phys. 16, 12715–12731 (2016).
Zhang, Y. et al. Insights into atmospheric oxidation processes by performing factor analyses on subranges of mass spectra. Atmos. Chem. Phys. 20, 5945–5961 (2020).
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014).
Troestl, J. et al. The role of low-volatility organic compounds in initial particle growth in the atmosphere. Nature 533, 527–531 (2016).
Kürten, A., Rondo, L., Ehrhart, S. & Curtius, J. Calibration of a chemical ionization mass spectrometer for the measurement of gaseous sulfuric acid. J. Phys. Chem. A 116, 6375–6386 (2012).
Heinritzi, M. et al. Characterization of the mass-dependent transmission efficiency of a CIMS. Atmos. Meas. Tech. 9, 1449–1460 (2016).
Zhang, Y. et al. A novel approach for simple statistical analysis of high-resolution mass spectra. Atmos. Meas. Tech. 12, 3761–3776 (2019).
Liu, Y. et al. Formation of condensable organic vapors from anthropogenic and biogenic volatile organic compounds (VOCs) is strongly perturbed by NOx in eastern China. Atmos. Chem. Phys. 21, 14789–14814 (2021).
Xu, Z. N. et al. Multifunctional products of isoprene oxidation in polluted atmosphere and their contribution to SOA. Geophys. Res. Lett. 48, e2020GL089276 (2021).
Bianchi, F. et al. Highly oxygenated organic molecules (HOM) from gas-phase autoxidation involving peroxy radicals: a key contributor to atmospheric aerosol. Chem. Rev. 119, 3472–3509 (2019).
Wang, M. et al. Photo-oxidation of aromatic hydrocarbons produces low-volatility organic compounds. Environ. Sci. Technol. 54, 7911–7921 (2020).
Dang, C. et al. The effect of structure and isomerism on the vapor pressures of organic molecules and its potential atmospheric relevance. Aerosol Sci. Tech. 53, 1040–1055 (2019).
Cheng, X. et al. Secondary production of gaseous nitrated phenols in polluted urban environments. Environ. Sci. Technol. 55, 4410–4419 (2021).
Wang, Z., Zhang, J., Zhang, L., Liang, Y. & Shi, Q. Characterization of nitroaromatic compounds in atmospheric particulate matter from Beijing. Atmos. Environ. 246, 118046 (2021).
Wang, Z. et al. Efficient alkane oxidation under combustion engine and atmospheric conditions. Commun. Chem. 4, 18 (2021).
Molteni, U. et al. Formation of highly oxygenated organic molecules from aromatic compounds. Atmos. Chem. Phys. 18, 1909–1921 (2018).
Garmash, O. et al. Multi-generation OH oxidation as a source for highly oxygenated organic molecules from aromatics. Atmos. Chem. Phys. 20, 515–537 (2020).
Wang, Y. et al. Oxygenated products formed from OH-initiated reactions of trimethylbenzene: autoxidation and accretion. Atmos. Chem. Phys. 20, 9563–9579 (2020).
Simon, M. et al. Molecular understanding of new-particle formation from α-pinene between −50 and +25 °C. Atmos. Chem. Phys. 20, 9183–9207 (2020).
Wang, S., Wu, R., Berndt, T., Ehn, M. & Wang, L. Formation of highly oxidized radicals and multifunctional products from the atmospheric oxidation of alkylbenzenes. Environ. Sci. Technol. 51, 8442–8449 (2017).
Mehra, A. et al. Evaluation of the chemical composition of gas- and particle-phase products of aromatic oxidation. Atmos. Chem. Phys. 20, 9783–9803 (2020).
Donahue, N. M., Epstein, S. A., Pandis, S. N. & Robinson, A. L. A two-dimensional volatility basis set: 1. Organic-aerosol mixing thermodynamics. Atmos. Chem. Phys. 11, 3303–3318 (2011).
Wang, L., Wu, R. & Xu, C. Atmospheric oxidation mechanism of benzene. Fates of alkoxy radical intermediates and revised mechanism. J. Phys. Chem. A 117, 14163–14168 (2013).
Pankow, J. F. & Asher, W. E. SIMPOL.1: a simple group contribution method for predicting vapor pressures and enthalpies of vaporization of multifunctional organic compounds. Atmos. Chem. Phys. 8, 2773–2796 (2008).
Mohr, C. et al. Molecular identification of organic vapors driving atmospheric nanoparticle growth. Nat. Commun. 10, 4442 (2019).
Li, Y., Pöschl, U. & Shiraiwa, M. Molecular corridors and parameterizations of volatility in the chemical evolution of organic aerosols. Atmos. Chem. Phys. 16, 3327–3344 (2016).
Tan, Z. et al. Wintertime photochemistry in Beijing: observations of ROx radical concentrations in the North China Plain during the BEST-ONE campaign. Atmos. Chem. Phys. 18, 12391–12411 (2018).
Lu, K. et al. Fast photochemistry in wintertime haze: consequences for pollution mitigation strategies. Environ. Sci. Technol. 53, 10676–10684 (2019).
Duan, J. et al. Summertime and wintertime atmospheric processes of secondary aerosol in Beijing. Atmos. Chem. Phys. 20, 3793–3807 (2020).
Huang, X. et al. Enhanced secondary pollution offset reduction of primary emissions during COVID-19 lockdown in China. Natl Sci. Rev. 8, nwaa137 (2021).
Tilmes, S. et al. Climate forcing and trends of organic aerosols in the Community Earth System Model (CESM2). J. Adv. Model. Earth Syst. 11, 4323–4351 (2019).
Zhang, F. et al. An unexpected catalyst dominates formation and radiative forcing of regional haze. Proc. Natl Acad. Sci. USA 117, 3960–3966 (2020).
Huang, X. et al. Amplified transboundary transport of haze by aerosol-boundary layer interaction in China. Nat. Geosci. 13, 428–434 (2020).
Zhang, X. et al. Influence of vapor wall loss in laboratory chambers on yields of secondary organic aerosol. Proc. Natl Acad. Sci. USA 111, 5802–5807 (2014).
Kulmala, M. et al. Opinion: Gigacity—a source of problems or the new way to sustainable development. Atmos. Chem. Phys. 21, 8313–8322 (2021).
Odum, J. R. et al. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 30, 2580–2585 (1996).
Cocker, D. R. III., Mader, B. T., Kalberer, M., Flagan, R. C. & Seinfeld, J. H. The effect of water on gas-particle partitioning of secondary organic aerosol: II. m-xylene and 1,3,5-trimethylbenzene photooxidation systems. Atmos. Environ. 35, 6073–6085 (2001).
Song, C., Na, K. & Cocker, D. R. Impact of the hydrocarbon to NOx ratio on secondary organic aerosol formation. Environ. Sci. Technol. 39, 3143–3149 (2005).
Ng, N. L. et al. Secondary organic aerosol formation from m-xylene, toluene and benzene. Atmos. Chem. Phys. 7, 3909–3922 (2007).
Sato, K. et al. AMS and LC/MS analyses of SOA from the photooxidation of benzene and 1,3,5-trimethylbenzene in the presence of NOx: effects of chemical structure on SOA aging. Atmos. Chem. Phys. 12, 4667–4682 (2012).
Li, L., Tang, P., Nakao, S., Chen, C. L. & Cocker, D. R. III. Role of methyl group number on SOA formation from monocyclic aromatic hydrocarbons photooxidation under low-NOx conditions. Atmos. Chem. Phys. 16, 2255–2272 (2016).
Li, L., Tang, P., Nakao, S. & Cocker, D. R. III. Impact of molecular structure on secondary organic aerosol formation from aromatic hydrocarbon photooxidation under low-NOx conditions. Atmos. Chem. Phys. 16, 10793–10808 (2016).
Breitenlechner, M. et al. PTR3: an instrument for studying the lifecycle of reactive organic carbon in the atmosphere. Anal. Chem. 89, 5824–5831 (2017).
Hyttinen, N. et al. Modeling the charging of highly oxidized cyclohexene ozonolysis products using nitrate-based chemical ionization. J. Phys. Chem. A 119, 6339–6345 (2015).
Hyttinen, N. et al. Computational comparison of different reagent ions in the chemical ionization of oxidized multifunctional compounds. J. Phys. Chem. A 122, 269–279 (2018).
Riva, M. et al. Evaluating the performance of five different chemical ionization techniques for detecting gaseous oxygenated organic species. Atmos. Meas. Tech. 12, 2403–2421 (2019).
Kawamura, K. & Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: molecular distributions, sources and transformation. Atmos. Res. 170, 140–160 (2016).
Elm, J., Hyttinen, N., Lin, J. J., Kurtén, T. & Prisle, N. L. Strong even/odd pattern in the computed gas-phase stability of dicarboxylic acid dimers: implications for condensation thermodynamics. J. Phys. Chem. A 123, 9594–9599 (2019).
Donahue, N. M., Robinson, A. L., Stanier, C. O. & Pandis, S. N. Coupled partitioning, dilution, and chemical aging of semivolatile organics. Environ. Sci. Technol. 40, 2635–2643 (2006).
Yang, L. et al. Toward building a physical proxy for gas-phase sulfuric acid concentration based on its budget analysis in polluted Yangtze River Delta, East China. Environ. Sci. Technol. 55, 6665–6676 (2021).
Seinfeld, J. H. & Pandis, S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change (Wiley, 2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) project (92044301, 41875175, 42075101, 21806108, 91744204 and 22188102), the Jiangsu Provincial Collaborative Innovation Center of Climate Change, Samsung PM2.5 SRP, the Research Grants Council of Hong Kong Special Administrative Region (grants nos. T24/504/17-N and 15265516), the Shanghai Rising-Star Program (19QB1402900) and the US National Science Foundation (AGS1801897). K.R.D. acknowledges support by the Swiss National Science Foundation mobility grant P2EZP2_181599. We thank Y. Liu for processing aerosol optical depth data. The Hong Kong team would like to acknowledge the HKPolyU University Research Facility in Chemical and Environmental Analysis (UCEA) for equipment support, and the Hong Kong Environmental Protection Department for providing access to the team to conduct measurements at Cape D’Aguilar Supersite AQMS and for sharing the trace gas, PM2.5 and VOCs data at the Supersite.
Author information
Authors and Affiliations
Contributions
W.N., C. Yan, D.D.H., Z.W., A.D., J.J. and M.K. designed the study. W.N., C. Yan, D.D.H., Z.W., Yuliang Liu, X. Qiao, Y.G., L.T. and P.Z. analysed the data. W.N., C. Yan, D.D.H., Z.W., A.D., J.J., M.E. and N.M.D. wrote the manuscript. Yuliang Liu, Zhengning Xu, Y. Li, X. Qiao, Y.G. and P.Z. collected other research materials. All authors participated in relevant scientific discussion and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Jacqueline Hamilton, Ru-Jin Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Xujia Jiang and Tom Richardson, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Time series of observed OOMs and related parameters in (a) Beijing, (b) Nanjing, (c) Shanghai, and (d) Hong Kong.
Variables include UVB (JO1D in Shanghai), Temperature, RH, NOx, O3, primary organic aerosol (POA), secondary organic aerosol (SOA), total OOMs and nitrophenols.
Extended Data Fig. 2 Correlation of OOMs measured by nitrate- and iodide- CIMS (A-F), C5-10 CHO OOMs with different number of oxygen (G) C5-10 CHO OOMs and (H) C5-10 CHON OOMs in Beijing.
Time period of data is from 1st to 28th January 2020. The numbers in the brackets are the corresponding number of common species that can be detected by both instruments. R denotes the person correlation coefficient.
Extended Data Fig. 3 Overview of the PMF results.
(a) comparison of PMF results from the four different cities; (b) an example of one of the common factors: profile and diurnal variation of common factor 6 (night-time monoterpene factor). The dark yellow dots in the diurnal plot represent the mean value; the line and the shaded area represent 50th percentile and 25th−75th percentile, respectively. (c) mass defect plot of common factor 6 in Nanjing.
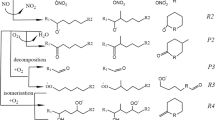
Extended Data Fig. 4 A decision-tree based workflow.
A decision-tree based workflow to identify the precursors of detected OOM.
Extended Data Fig. 5 The accuracy of workflow tested by known OOM peak lists of (a) Nitrate-CIMS and (b) I-CIMS from laboratory experiments.
Superscripts 1, 2, 3 in panel (a) refer to (1) Molteni et al., 2018, (2) Garmash et al., 2020, and (3) Wang et al., 2021. There are 4 different experiments for the oxidation of benzene: (*) Experiment in a flow reactor, (**) Experiment with low OH/VOC in the JPAC chamber, (***) Experiment with high OH/VOC in the JPAC chamber, and (****) Experiment affected by NOx in the JPAC chamber. Peak lists used in panel (b) are from Mehra et al., 2020.
Extended Data Fig. 6 The distributions of the total observed OOMs (upper panel), aliphatic-OOMs (middle panel) and aromatic-OOMs (lower panel) grouped by (a) the numbers of carbon (nC), (b) effective oxygen (nO-eff), (c) nitrogen (nN) and (d) double bond equivalent (DBE) in four different cities.
Columns in blue, green, orange and purple represent Beijing, Nanjing, Shanghai and Hong Kong, respectively.
Extended Data Fig. 7 Volatility distribution of observed OOMs in 4 megacities at (a) observed actual temperature, and (b) 300 K.
Columns in blue, green, orange and purple represent Beijing, Nanjing, Shanghai and Hong Kong, respectively.
Extended Data Fig. 8 OOMs condensation flux as a dependent of PM2.5 in (a) Beijing, (b) Nanjing, (c) Shanghai, and (d) Hong Kong.
Aliphatic-OOMs in red, aromatic-OOMs in blue, isoprene-OOMs in bright yellow, monoterpene-OOMs in dark yellow and undistinguished OOMs in grey.
Extended Data Fig. 9 Correlation between detected OOMs from different precursor classes and PM2.5 in Beijing, Nanjing, Shanghai and Hong Kong.
Aliphatic-OOMs in red, aromatic-OOMs in blue, isoprene-OOMs in bright yellow, monoterpene-OOMs in dark yellow and undistinguished OOMs in grey.
Extended Data Fig. 10 Case showing the selection of SOA formation episode.
A stable weather condition is critical for selecting SOA formation episodes to exclude the influence of air mass changes. Four parameters, including wind speed, wind direction, boundary layer height and water concentrations, are used for the judgement. For one sampling period, when these four parameters kept stable, it can be identified as a SOA episode. These selected SOA episodes mostly concentrated around noontime or night-time time when the boundary layer was stable. In total, 16 episodes in Beijing, 19 episodes in Nanjing, 8 episodes in Shanghai, and 18 episodes in Hong Kong were selected for further analysis. We also test the potential uncertainties from case selection by varying the start- and end-point of the case. Cases with the uncertainties of d(SOA)/dt higher than 30% are excluded.
Supplementary information
Supplementary Information
Supplementary information of sampling sites and measurements, Figs. 1–3 and Tables 1–3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Molecular composition data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
About this article
Cite this article
Nie, W., Yan, C., Huang, D.D. et al. Secondary organic aerosol formed by condensing anthropogenic vapours over China’s megacities. Nat. Geosci. 15, 255–261 (2022). https://doi.org/10.1038/s41561-022-00922-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-00922-5
This article is cited by
-
One-year observation of the mixing states of oxygenated organics-containing single particles in Guangzhou, China
Frontiers of Environmental Science & Engineering (2024)
-
Molecular rearrangement of bicyclic peroxy radicals is a key route to aerosol from aromatics
Nature Communications (2023)
-
Aerosol high water contents favor sulfate and secondary organic aerosol formation from fossil fuel combustion emissions
npj Climate and Atmospheric Science (2023)
-
Increased night-time oxidation over China despite widespread decrease across the globe
Nature Geoscience (2023)
-
Response of organic aerosol characteristics to emission reduction in Yangtze River Delta region
Frontiers of Environmental Science & Engineering (2023)