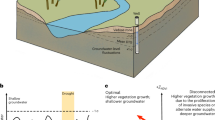
Abstract
Water potential directly controls the function of leaves, roots and microbes, and gradients in water potential drive water flows throughout the soil–plant–atmosphere continuum. Notwithstanding its clear relevance for many ecosystem processes, soil water potential is rarely measured in situ, and plant water potential observations are generally discrete, sparse, and not yet aggregated into accessible databases. These gaps limit our conceptual understanding of biophysical responses to moisture stress and inject large uncertainty into hydrologic and land-surface models. Here, we outline the conceptual and predictive gains that could be made with more continuous and discoverable observations of water potential in soils and plants. We discuss improvements to sensor technologies that facilitate in situ characterization of water potential, as well as strategies for building new networks that aggregate water potential data across sites. We end by highlighting novel opportunities for linking more representative site-level observations of water potential to remotely sensed proxies. Together, these considerations offer a road map for clearer links between ecohydrological processes and the water potential gradients that have the ‘potential’ to substantially reduce conceptual and modelling uncertainties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The FLUXNET tower data appearing in Fig. 3 are from the FLUXNET 2015 dataset (https://doi.org/10.18140/FLX/1440186 for SD-Dem, https://doi.org/10.18140/FLX/1440071 for US-HA1 and https://doi.org/10.18140/FLX/1440160 for FI-SOD). The AmeriFlux tower data appearing in Fig. 4 are available from the AmeriFlux network (https://doi.org/10.17190/AMF/1246080 for US-MMS, https://doi.org/10.17190/AMF/1246081 for US-MOz, https://doi.org/10.17190/AMF/1246104 for US-SRM and https://doi.org/10.17190/AMF/1245971 for US-TON).
Code availability
The HYDRUS 1D programme used to create the results of Fig. 2e–g is available for public download from https://www.pc-progress.com/en/Default.aspx?hydrus-1d. A reference version of the ORCHIDEE land-surface model, used for Fig. 3, is available at https://orchidee.ipsl.fr/. Details on the parameterizations of these models are presented in the Supplementary Information.
Reference
Brutsaert, W. Hydrology: An Introduction (Cambridge Univ. Press, 2005).
Philip, J. Plant water relations: some physical aspects. Annu. Rev. Plant Physiol. 17, 245–268 (1966).
Ghezzehei, T. A., Sulman, B., Arnold, C. L., Bogie, N. A. & Berhe, A. A. On the role of soil water retention characteristic on aerobic microbial respiration. Biogeosciences 16, 1187–1209 (2019).
Boyer, J. Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol. 46, 236–239 (1970).
Jarvis, P. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Phil. Trans. R. Soc. Lond. B 273, 593–610 (1976).
Choat, B. et al. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755 (2012).
Tyree, M. T. & Sperry, J. S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Biol. 40, 19–36 (1989).
Whalley, W., Ober, E. & Jenkins, M. J. J. Measurement of the matric potential of soil water in the rhizosphere. J. Exp. Biol. 64, 3951–3963 (2013).
Yu, H., Yang, P. & Lin, H. Spatiotemporal patterns of soil matric potential in the Shale Hills Critical Zone Observatory. Vadose Zone J. https://doi.org/10.2136/vzj2014.11.0167 (2015).
Campbell, G. S. A simple method for determining unsaturated conductivity from moisture retention data. Soil Sci. 117, 311–314 (1974).
van Genuchten, M. T. A closed‐form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 44, 892–898 (1980).
Dorigo, W. et al. The International Soil Moisture Network: a data hosting facility for global in situ soil moisture measurements. Hydrol. Earth Syst. Sci. 15, 1675–1698 (2011).
Scott, B. L. et al. New soil property database improves Oklahoma Mesonet soil moisture estimates. J. Atmos. Ocean. Technol. 30, 2585–2595 (2013).
Campbell, G. S. Soil water potential measurement: an overview. Irrig. Sci. 9, 265–273 (1988).
Van Looy, K. et al. Pedotransfer functions in Earth system science: challenges and perspectives. Rev. Geophys. 55, 1199–1256 (2017).
Clapp, R. B. & Hornberger, G. M. Empirical equations for some soil hydraulic properties. Water Resour. Res. 14, 601–604 (1978).
Cosby, B., Hornberger, G., Clapp, R. & Ginn, T. A statistical exploration of the relationships of soil moisture characteristics to the physical properties of soils. Water Resour. Res. 20, 682–690 (1984).
Zhang, Y. & Schaap, M. G. Weighted recalibration of the Rosetta pedotransfer model with improved estimates of hydraulic parameter distributions and summary statistics (Rosetta3). J. Hydrol. 547, 39–53 (2017).
Fatichi, S. et al. Soil structure is an important omission in Earth system models. Nat. Commun. 11, 522 (2020).
Ghezzehei, T. A. & Albalasmeh, A. A. Spatial distribution of rhizodeposits provides built-in water potential gradient in the rhizosphere. Ecol. Modell. 298, 53–63 (2015).
Leung, A. K., Garg, A. & Ng, C. W. W. Effects of plant roots on soil-water retention and induced suction in vegetated soil. Eng. Geol. 193, 183–197 (2015).
Caplan, J. S. et al. Decadal-scale shifts in soil hydraulic properties as induced by altered precipitation. Sci. Adv. 5, eaau6635 (2019).
Peña-Sancho, C., López, M., Gracia, R. & Moret-Fernández, D. Effects of tillage on the soil water retention curve during a fallow period of a semiarid dryland. Soil Res. 55, 114–123 (2017).
Stoof, C. R., Wesseling, J. G. & Ritsema, C. J. Effects of fire and ash on soil water retention. Geoderma 159, 276–285 (2010).
Gutmann, E. & Small, E. The effect of soil hydraulic properties vs. soil texture in land surface models. Geophys. Res. Lett. 32, L02402 (2005).
Weihermüller, L. et al. Choice of pedotransfer functions matters when simulating soil water balance fluxes. J. Adv. Model. Earth Syst. 13, e2020MS002404 (2021).
Shi, Y., Davis, K. J., Zhang, F. & Duffy, C. J. Evaluation of the parameter sensitivities of a coupled land surface hydrologic model at a critical zone observatory. J. Hydrometeorol. 15, 279–299 (2014).
Shi, Y., Davis, K. J., Zhang, F., Duffy, C. J. & Yu, X. J. Parameter estimation of a physically-based land surface hydrologic model using an ensemble Kalman filter: a multivariate real-data experiment. Adv. Water Res. 83, 421–427 (2015).
Shi, Y. et al. Simulating high‐resolution soil moisture patterns in the Shale Hills watershed using a land surface hydrologic model. Hydrol. Process. 29, 4624–4637 (2015).
Sobol, I. M. Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math. Comput. Simul. 55, 271–280 (2001).
Boucher, O. et al. Presentation and evaluation of the IPSL‐CM6A‐LR climate model. J. Adv. Model. Earth Syst. 12, e2019MS002010 (2020).
Lurton, T. et al. Implementation of the CMIP6 forcing data in the IPSL‐CM6A‐LR model. J. Adv. Model. Earth Syst. 12, e2019MS001940 (2020).
Green, J. K. et al. Large influence of soil moisture on long-term terrestrial carbon uptake. Nature 565, 476–479 (2019).
Jung, M. et al. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature 467, 951–954 (2010).
Novick, K. A. et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Change 6, 1023–1027 (2016).
Feldman, A. F., Short Gianotti, D. J., Trigo, I. F., Salvucci, G. D. & Entekhabi, D. Satellite‐based assessment of land surface energy partitioning–soil moisture relationships and effects of confounding variables. Water Resour. Res. 55, 10657–10677 (2019).
Stocker, B. D. et al. Quantifying soil moisture impacts on light use efficiency across biomes. N. Phytol. 218, 1430–1449 (2018).
Baldocchi, D. D., Xu, L. & Kiang, N. How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak–grass savanna and an annual grassland. Agric. For. Meteorol. 123, 13–39 (2004).
Trugman, A. T., Anderegg, L. D., Shaw, J. D. & Anderegg, W. R. Trait velocities reveal that mortality has driven widespread coordinated shifts in forest hydraulic trait composition. Proc. Natl Acad. Sci. USA 117, 8532–8538 (2020).
McDowell, N. et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? N. Phytol. 178, 719–739 (2008).
Martínez-Vilalta, J. et al. Towards a statistically robust determination of minimum water potential and hydraulic risk in plants. New Phytol. 232, 404–417 (2021).
Taiz, L., Zeiger, E., Møller, I. M. & Murphy, A. Plant Physiology and Development 6th edn (Sinauer Associates, 2015).
Scholander, P. F., Bradstreet, E. D., Hemmingsen, E. & Hammel, H. Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science 148, 339–346 (1965).
Martínez‐Vilalta, J., Poyatos, R., Aguadé, D., Retana, J. & Mencuccini, M. A new look at water transport regulation in plants. N. Phytol. 204, 105–115 (2014).
Grossiord, C. et al. Plant responses to rising vapor pressure deficit. N. Phytol. 226, 1550–1566 (2020).
Matheny, A. M. et al. Observations of stem water storage in trees of opposing hydraulic strategies. Ecosphere https://doi.org/10.1890/es15-00170.1 (2015).
Wood, J. D., Knapp, B. O., Muzika, R.-M., Stambaugh, M. C. & Gu, L. The importance of drought–pathogen interactions in driving oak mortality events in the Ozark Border Region. Environ. Res. Lett. 13, 015004 (2018).
Hinckley, T. M., Lassoie, J. P. & Running, S. W. Temporal and spatial variations in the water status of forest trees. For. Sci. 24, a0001–z0001 (1978).
Marks, C. O. & Lechowicz, M. J. The ecological and functional correlates of nocturnal transpiration. Tree Physiol. 27, 577–584 (2007).
O’Keefe, K. & Nippert, J. B. Drivers of nocturnal water flux in a tallgrass prairie. Funct. Ecol. 32, 1155–1167 (2018).
Donovan, L., Linton, M. & Richards, J. Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129, 328–335 (2001).
Kannenberg, S. A. et al. Opportunities, challenges and pitfalls in characterizing plant water‐use strategies. Funct. Ecol. 36, 24–37 (2022).
Oliveira, R. S. et al. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytol. 230, 904–923 (2021).
Feng, X. et al. Beyond isohydricity: the role of environmental variability in determining plant drought responses. Plant Cell Environ. 42, 1104–1111 (2019).
Guo, J. S., Hultine, K. R., Koch, G. W., Kropp, H. & Ogle, K. Temporal shifts in iso/anisohydry revealed from daily observations of plant water potential in a dominant desert shrub. N. Phytol. 225, 713–726 (2020).
Hochberg, U., Rockwell, F. E., Holbrook, N. M. & Cochard, H. Iso/anisohydry: a plant–environment interaction rather than a simple hydraulic trait. Trends Plant Sci. 23, 112–120 (2018).
Novick, K. A., Konings, A. G. & Gentine, P. Beyond soil water potential: an expanded view on isohydricity including land–atmosphere interactions and phenology. Plant Cell Environ. 42, 1802–1815 (2019).
McCulloh, K. A. et al. A dynamic yet vulnerable pipeline: integration and coordination of hydraulic traits across whole plants. Plant Cell Environ. 42, 2789–2807 (2019).
Kennedy, D. et al. Implementing plant hydraulics in the Community Land Model, version 5. J. Adv. Model. Earth Syst. 11, 485–513 (2019).
Mirfenderesgi, G., Matheny, A. M. & Bohrer, G. Hydrodynamic trait coordination and cost–benefit trade‐offs throughout the isohydric–anisohydric continuum in trees. Ecohydrology 12, e2041 (2019).
Xu, X., Medvigy, D., Powers, J. S., Becknell, J. M. & Guan, K. Diversity in plant hydraulic traits explains seasonal and inter‐annual variations of vegetation dynamics in seasonally dry tropical forests. N. Phytol. 212, 80–95 (2016).
De Kauwe, M. G. et al. Do land surface models need to include differential plant species responses to drought? Examining model predictions across a mesic-xeric gradient in Europe. Biogeosciences 12, 7503–7518 (2015).
Meinzer, F. C. et al. Converging patterns of uptake and hydraulic redistribution of soil water in contrasting woody vegetation types. Tree Physiol. 24, 919–928 (2004).
Scott, R. L., Cable, W. L. & Hultine, K. R. The ecohydrologic significance of hydraulic redistribution in a semiarid savanna. Water Resour. Res. 44, W02440 (2008).
Tyree, M. T. & Ewers, F. W. The hydraulic architecture of trees and other woody plants. N. Phytol. 119, 345–360 (1991).
Johnson, D. M. et al. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol. 36, 983–993 (2016).
Lehto, T. & Zwiazek, J. J. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21, 71–90 (2011).
Bezerra-Coelho, C. R., Zhuang, L., Barbosa, M. C., Soto, M. A. & Van Genuchten, M. T. Further tests of the HYPROP evaporation method for estimating the unsaturated soil hydraulic properties. J. Hydrol. Hydromech. 66, 161–169 (2018).
Wullschleger, S., Dixon, M. & Oosterhuis, D. Field measurement of leaf water potential with a temperature‐corrected in situ thermocouple psychrometer. Plant Cell Environ. 11, 199–203 (1988).
Holtzman, N. M. et al. L-band vegetation optical depth as an indicator of plant water potential in a temperate deciduous forest stand. Biogeosciences 18, 739–753 (2021).
Nagy, R. C. et al. Harnessing the NEON data revolution to advance open environmental science with a diverse and data‐capable community. Ecosphere 12, e03833 (2021).
Novick, K. A. et al. The AmeriFlux network: a coalition of the willing. Agric. For. Meteorol. 249, 444–456 (2018).
Baldocchi, D. ‘Breathing’ of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 56, 1–26 (2008).
Poyatos, R. et al. Global transpiration data from sap flow measurements: the SAPFLUXNET database. Earth Syst. Sci. Data 13, 2607–2649 (2021).
Jackson, T. & Schmugge, T. Vegetation effects on the microwave emission of soils. Remote Sens. Environ. 36, 203–212 (1991).
Konings, A. G., Rao, K. & Steele‐Dunne, S. C. Macro to micro: microwave remote sensing of plant water content for physiology and ecology. N. Phytol. 223, 1166–1172 (2019).
Konings, A. G. et al. Detecting forest response to droughts with global observations of vegetation water content. Glob. Change Biol. https://doi.org/10.1111/gcb.15872 (2021).
Momen, M. et al. Interacting effects of leaf water potential and biomass on vegetation optical depth. J. Geophys. Res. Biogeosci. 122, 3031–3046 (2017).
Simunek, J., Van Genuchten, M. T. & Sejna, M. The HYDRUS-1D Software Package for Simulating the One-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media (Dept Environ. Sci. Univ. California Riverside, 2005).
Naylor, S., Letsinger, S., Ficklin, D., Ellett, K. & Olyphant, G. A hydropedological approach to quantifying groundwater recharge in various glacial settings of the mid‐continental USA. Hydrol. Process. 30, 1594–1608 (2016).
Urbanski, S. et al. Factors controlling CO2 exchange on timescales from hourly to decadal at Harvard Forest. J. Geophys. Res. Biogeosci. 112, G02020 (2007).
Thum, T. et al. Parametrization of two photosynthesis models at the canopy scale in a northern boreal Scots pine forest. Tellus B 59, 874–890 (2007).
Ardö, J., Mölder, M., El-Tahir, B. A. & Elkhidir, H. A. M. Seasonal variation of carbon fluxes in a sparse savanna in semi arid Sudan. Carbon Balance Manage. 3, 7 (2008).
Roman, D. T. et al. The role of isohydric and anisohydric species in determining ecosystem-scale response to severe drought. Oecologia 179, 641–654 (2015).
Fu, C. et al. Combined measurement and modeling of the hydrological impact of hydraulic redistribution using CLM4.5 at eight AmeriFlux sites. Hydrol. Earth Syst. Sci. 20, 2001–2018 (2016).
Liang, J. et al. Evaluating the E3SM land model version 0 (ELMv0) at a temperate forest site using flux and soil water measurements. Geosci. Model Dev. 12, 1601–1612 (2019).
Herman, J. & Usher, W. SALib: an open-source Python library for sensitivity analysis. J. Open Source Softw. https://doi.org/10.21105/joss.00097 (2017).
Acknowledgements
K.A.N. acknowledges support from NSF (DEB, grant 1552747) and the AmeriFlux Management Project via the US Department of Energy, Office of Science Lawrence Berkeley National Laboratory. A.G.K. was supported by NASA Terrestrial Ecology (award 80NSSC18K0715). J.D.W. acknowledges support from the US Department of Energy, Office of Science, through Oak Ridge National Laboratory’s Terrestrial Ecosystem Science Focus Area. K.J.D. and Y.S. were supported by National Science Foundation grant EAR 1331726 (S. Brantley) for the Susquehanna Shale Hills Critical Zone Observatory.
Author information
Authors and Affiliations
Contributions
K.A.N. conceived of the study with substantial input from D.L.F., A.G.K., K.J.D., T.A.G., R.L.S., B.N.S., Y.S. and N.M. Data analyses were performed by K.A.N., T.A.G., D.L.F. and N.R., who also created the resulting figures. D.B., R.L.S., K.A.N. and J.D.W. contributed AmeriFlux data used in Fig. 4. All authors wrote the text and provided substantial conceptual input to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Christopher Still, Vincent Humphrey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Tables 1–4 and Fig. 1.
Rights and permissions
About this article
Cite this article
Novick, K.A., Ficklin, D.L., Baldocchi, D. et al. Confronting the water potential information gap. Nat. Geosci. 15, 158–164 (2022). https://doi.org/10.1038/s41561-022-00909-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-00909-2
This article is cited by
-
Plant responses to changing rainfall frequency and intensity
Nature Reviews Earth & Environment (2024)
-
A closer look at root water potential: experimental evidence based on drought stress of Chrysopogon zizanioides
Plant and Soil (2024)
-
Multistage coupling water-enabled electric generator with customizable energy output
Nature Communications (2023)
-
Cross-sectoral impacts of the 2018–2019 Central European drought and climate resilience in the German part of the Elbe River basin
Regional Environmental Change (2023)
-
Water as the often neglected medium at the interface between materials and biology
Nature Communications (2022)