Abstract
The biotic and abiotic controls on major shifts in atmospheric oxygen and the persistence of low-oxygen periods over a majority of Earth’s history remain under debate. Explanations of Earth’s stepwise pattern of oxygenation have mostly neglected the effect of changing diel illumination dynamics linked to daylength, which has increased through geological time due to Earth’s rotational deceleration caused by tidal friction. Here we used microsensor measurements and dynamic modelling of interfacial solute fluxes in cyanobacterial mats to investigate the effect of changing daylength on Precambrian benthic ecosystems. Simulated increases in daylength across Earth’s historical range boosted the diel benthic oxygen export, even when the gross photosynthetic production remained constant. This fundamental relationship between net productivity and daylength emerges from the interaction of diffusive mass transfer and diel illumination dynamics, and is amplified by metabolic regulation and microbial behaviour. We found that the resultant daylength-driven surplus organic carbon burial could have shaped the increase in atmospheric oxygen that occurred during the Great and Neoproterozoic Oxidation Events. Our suggested mechanism, which links the coinciding increases in daylength and atmospheric oxygen via enhanced net productivity, reveals a possible contribution of planetary mechanics to the evolution of Earth’s biology and geochemistry.
Similar content being viewed by others
Main
The rise of free oxygen (O2) in the Earth’s atmosphere and oceans enabled the evolution of aerobic life1. Oxygenic photosynthesis (OP) in microbial mats was a substantial source of O2 for the Great Oxidation Event (GOE) ~2.4 billion years ago (Ga), during the stable low-O2 conditions that followed and for the Neoproterozoic Oxygenation Event (NOE) ~600 Ma (ref. 2). The biological3,4, tectonic5 and geochemical6,7 mechanisms that determined this stepwise pattern of oxygenation are still debated. Here we explore a previously unconsidered link between Earth’s oxygenation pattern and rotation rate, which decelerated over geological time due to tidal friction8,9. We establish a mechanistic link between daylength and export fluxes of solutes from microbial mats. Experimental measurements and models of Proterozoic cyanobacterial mat analogues show that longer daylength increases benthic O2 export, changes the balance between aerobic and anaerobic remineralization, and thus enhances the diel organic carbon (Corg) burial. We then investigated the remarkable similarity between the timing and pattern of increase in atmospheric O2 (\(p{{\rm{O}}_2}\) as a fraction of the present atmospheric level (PAL)) and daylength. We found that increases in daylength could plausibly have influenced Earth’s oxygenation, particularly around key oxidation events, and thus helped to pave the way for the evolution of plants and animals of the modern world.
Longer days increase net benthic O2 export fluxes
Earth’s rotation period is 24 hours at present, but may have been as low as 6 hours at ages older than 4 Ga (refs 8,10,11). Thus, daylength (that is, one rotational or diel period) and the illumination period may have increased more than threefold since the evolutionary origin of photosynthesis. This implies that the dynamics of illumination (rate of increase and decrease) within the diel period changed substantially. The rate of gross photosynthetic production (GPP) is governed by the instantaneous photon flux, irrespective of illumination dynamics and daylength. The net production rate, equivalent to diel Corg burial, is the result of this GPP and the rate of Corg consumption. As opposed to GPP, the net production of benthic ecosystems is expected to be influenced by changes in the illumination dynamics. In such systems, rates of net productivity are not only shaped by the instantaneous photon flux, but also by fluxes of metabolic substrates and products, which are governed by molecular diffusion. Thus, import, export and accumulation of metabolites should be sensitive to daylength due to the interaction between illumination dynamics and diffusive mass transfer.
We developed a modelled understanding of this interaction and first explored the implications for the export fluxes of the photosynthetic product O2. Our modelling framework12 formulates benthic ecosystems as diffusive–reactive systems with OP, anoxygenic photosynthesis (AP), aerobic respiration (Raero), sulfate reduction (anaerobic respiration (Ranaero)) and abiotic sulfide oxidation (SOX) (Extended Data Fig. 1 and Supplementary Video 1). Starting with a simple in silico mat with only OP and Raero, simulations with the same rate of GPP showed that longer days yield higher export fluxes, that is, an increase in O2 escape to the overlying water (Fig. 1a,b). The mechanism behind this relationship is apparent in the duration between the maxima of gross O2 production and export (Fig. 1a), which illustrates how mass transfer resistance delays the O2 escape. The export-limiting delay represents a smaller fraction of the day for longer daylengths. Longer days build up steeper O2 gradients and therefore higher fluxes, both upward and downward (Extended Data Fig. 2a). In thin mats, the latter would interact with the mat substrate (for example, pyrite), and possibly create a daylength-driven increase in preserved weathering signals13. The daylength-driven increase in O2 fluxes also alters the availability of O2 within the mat, boosts the fraction of produced Corg that escapes Raero and thus decreases remineralization efficiency (Fig. 1b). Consequently, daylength interacts with the net productivity of benthic systems, which relates to the short-term (that is, diel) Corg excess, and possibly the long-term Corg burial rate, a crucial determinant for the state of global \(p{{\rm{O}}_2}\) (refs 1,14). Overall, although GPP rates are unaffected by changes in daylength, Corg burial is modulated through the physics of molecular diffusion.
a, Depth-integrated gross OP (∫zOP, grey fill) in a pure OP system (‘OP no H2S’) under a water column with 25 µM O2 (\(p{{\rm{O}}_2}\) = 0.1) is identical for the two daylengths with the time of day viewed as a fraction of the diel period. However, the diel fraction during which export is tempered by mass transfer, illustrated by the duration between the maxima of photosynthesis (black dotted line) and export (blue and orange dotted lines), is sensitive to daylength. b, As longer days export more O2, less O2 is available for aerobic respiration (∫zRaero) in the mat, and therefore the burial of Corg increases with daylength. c, In the presence of anaerobic respiration by sulfur-reducing bacteria, both O2 and H2S export fluxes are modulated by daylength, even though the gross photosynthesis and sulfide production by anaerobic respiration are independent of the illumination dynamics in this simulation (‘OP SRB constant’). d, The diel Corg burial flux is defined here as the photosynthetically produced Corg that escapes both aerobic and anaerobic respiration (∫zOP – ∫zRaero – ∫zRanaero) and equals the difference between the export budgets of O2 and H2S (shown in Corg equivalents). The decrease of ∫zRaero compared with the diel budget in b is due to abiotic sulfide oxidation (SOX), an alternative sink of O2. As ∫zRanaero is set to be constant, the overall effect remains: mats export more O2 and retain more Corg in longer days.
Daylength increases O2 export more than reductant export
A more realistic scenario includes Ranaero as an additional sink of Corg, which we implemented in the form of sulfate reduction. Conceptually, sulfate and sulfide, the substrate and product, respectively, can be exchanged with any other redox couple, such as Fe(III)/Fe(II). We focus on the sulfur cycle because of the early evolutionary onset of sulfate reduction15 and because sulfide was transiently abundant in Precambrian coastal habitats16. We first chose a mat scenario with the Ranaero rate fixed to a constant value, to isolate the effect of daylength on diffusion-driven dynamics of sulfide export (Fig. 1c). Like O2 fluxes, the reductant export fluxes are shaped by molecular diffusion and rates of production and consumption within the mat (Ranaero and SOX). The latter is an additional sink of O2 and is thus competitive with Raero. Consequently, the Corg that escapes both anaerobic and aerobic remineralization can be represented as the difference between the O2 and H2S export fluxes (Fig. 1c,d and Extended Data Fig. 1). As both fluxes increase with daylength, the rates of SOX decrease. Owing to this moderating effect of SOX, Raero is less sensitive to daylength than it is in a reductant-free scenario. The effect of an increased O2 export on the diel Corg burial rate is therefore counteracted, but not overwhelmed, by concomitant increases in H2S export (Fig. 1d). To further explore if the increase of diel Corg burial is maintained when Ranaero is affected by local solute dynamics, we implemented sulfate-reducing bacteria (SRB) that are inhibited by O2 and H2S, as observed in modern mats17,18,19. Across a range of inhibition strengths, diel Corg burial consistently increases with daylength (Fig. 2 and Extended Data Fig. 3).
The effect of daylength is explored for mats with different metabolic repertoires across a range of O2 boundary conditions (0–1 \(p{{\rm{O}}_2}\) in the shaded areas; Extended Data Figs. 3 and 5). a, O2 export fluxes consistently increase across all the metabolic scenarios and boundary conditions. Measured O2 export from cyanobacterial mats with chemosynthetic competition (‘MIS empirical’) provides empirical confirmation of the dependency of fluxes on daylength. b, Owing to the O2 export flux modulation, the diel Corg burial also consistently increases with daylength despite the counteracting effect of an increasing H2S export for scenarios with Ranaero (Fig. 1). Fluxes are shown relative to the values of each scenario at 18 h, which corresponds to daylength before the GOE. Metabolic scenarios explored were: purely aerobic systems with only OP and no Ranaero (‘OP no H2S’), systems with constant Ranaero that is not affected by changing diel illumination dynamics (‘OP SRB constant’), systems with metabolically complex Ranaero that responds to local changes in O2 and sulfide, which are governed by illumination dynamics (‘OP SRB’) and mat systems with both AP and OP (‘OP SRB + AP’). Systems with sulfide-inhibited OP exhibited a steepness of daylength dependency out of scale, and are shown in Extended Data Fig. 8. For the Ranaero scenario with a modest inhibition by O2 and H2S (‘OP SRB’), we additionally explored a wide range of plausible GPP levels (‘OP SRB net production’). Notably, the effect of variations in GPP on burial is less pronounced than that on O2 export.
Given that the Earth’s redox landscape changed along with daylength through geological time, we studied the sensitivity of daylength-driven diel Corg burial to reductant and O2 availability in the water column. Diel O2 export and Corg burial fluxes increased with daylength across all O2 boundary conditions (Fig. 2). Increasing O2 in the water column had a strong enhancing effect on Raero and negative effect on O2 export fluxes. For the diel Corg burial rate, determined by both Raero and Ranaero, the effect of O2 levels in the water column was variable (Extended Data Fig. 3). Depending on the inhibition strength of O2 on Ranaero, Corg burial either increases or decreases with the O2 boundary conditions. Given that Ranaero is O2 sensitive, the negative impact of \(p{{\rm{O}}_2}\) is overwhelmed by the positive effect of daylength enhancing diel benthic Corg burial over Earth’s history.
To explore the interactions between reductant availability and Corg burial with daylength, we also included AP using H2S as an electron donor that competes with OP for the contribution to GPP based on mat-intrinsic reductant and light levels20 (Extended Data Fig. 4). With AP present in mats, the fraction of the day that exports O2 is reduced for shorter daylengths because of the time required for AP to deplete the local sulfide concentration below the thresholds that allow OP to occur (Extended Data Fig. 5). Although the total GPP (AP + OP) is unaffected by daylength, the fraction of the day during which O2 is produced decreases markedly with daylength. Concomitantly, Ranaero decreases with daylength due to the increasing inhibition by O2. Although counterintuitive, Raero decreases with daylength because SOX becomes more competitive for O2 due to the increasing fractions of the day with O2 production. Consequently, across a range of sulfide and O2 levels in the overlying water, we found that the Corg burial of systems with AP is more steeply modulated by daylength compared with that for communities with only OP (Fig. 2). Interestingly, the dependency of the diel Corg burial on the reductant boundary condition was negligible compared to that on daylength. Therefore, the metabolic repertoire, rather than the water column redox conditions, shapes the response of benthic systems to daylength in terms of diel Corg burial.
Overall, we show that the net productivity, that is, the short-term Corg burial of benthic ecosystems, and thus a crucial determinant of the source strength for global \(p{{\rm{O}}_2}\), can increase with daylength over Earth’s age without assuming a decrease in the global O2 sink strength or an increase in GPP. However, the range of global GPP probably varied substantially, for example, due to new evolutionary avenues of primary production21, redox and phosphate oscillations related to weathering during the ‘boring billion’ years6, continental reconfiguration22, long-term changes in insolation23 or even daylength-related changes in ocean circulation and nutrient supply by upwelling24. We therefore evaluated the sensitivity of daylength-driven increases in benthic O2 export to the rates of GPP. As expected, O2 export displays a steep dependency on GPP, but daylength-driven changes in Corg burial are substantially less sensitive to GPP (Fig. 2). This implies that the areal coverage of benthic habitats rather than the evolution of GPP by the inhabitants is of greatest relevance for daylength-driven effects.
Empirical verification of the daylength effect
To reify the concept of export fluxes being modulated by daylength, we measured rates of photosynthesis and O2 export in cyanobacterial mats from the Middle Island Sinkhole (MIS), an extant analogue of Proterozoic mats under low-O2 conditions25. Net O2 production consistently occurred only after extended exposure time to light (Fig. 3a). White sulfur-oxidizing bacteria (SOB) atop the mat during night and morning reduced the light availability for photosynthesis26. The cyanobacteria exclusively performed AP, and thereby depleted the sulfide underneath the SOB layer (Fig. 3b,c). The sudden onset of O2 production during the phase of high light in the early afternoon (Fig. 3d) coincided with the downward migration of the light-reflecting white SOB, which was probably induced by depletion of sulfide by cyanobacterial AP (Extended Data Fig. 6a). This migration was triggered only after an additional lag of 1–8 hours after sulfide depletion, depending on the O2 and sulfide levels in the overlying water (Extended Data Fig. 6b). The ensuing exposure of cyanobacteria to a higher photon flux at the mat surface resulted in high rates of OP and AP and the onset of net O2 export. As the vertical structure of the mat persisted during subsequent lower light intensities, rates of both OP and AP remained high (Fig. 3e).
a, Vertical profiles of O2 in one of the mats are shown as a colour-coded map over the simulated diel light cycle and illustrate a delay in the net photosynthetic O2 production. The light intensity at the mat surface is indicated as I (µmol photons m−2 s−1). b–e, Depth profiles of O2 (open blue circles), total sulfide (calculated from [H2S] and pH; open black squares), gross OP and sulfide-driven AP (filled blue circles and black squares, respectively) (n = 3–4 for measurements at each depth) at the selected time points (b–e) shown in a reveal the cascade of structural and metabolic changes that result in a delayed O2 release. The vertical mat structure is represented as shaded areas. Although sulfide was depleted by AP upon illumination (b and c), OP occurred despite extended illumination only when the mat structure changed (d and e). The resultant delay in net O2 production was consistently observed in four separate mat samples exposed to similar water-column conditions, and throughout seven additional mat samples exposed to a wide range of sulfide and O2 levels in the water column (Extended Data Fig. 6). The magnitude of the fluxes and the duration of the delays were dependent on the specific sample and on the water-column redox conditions. f, The momentary export flux across the benthic interface was measured over 12–24 h simulated daylengths in one mat sample exposed to 1 µM O2 from the water column in the absence of sulfide (Extended Data Fig. 7). The resultant diel O2 export is shown in the key and increased with daylength.
The chemosynthetic SOB modulated the locally availability of light for cyanobacteria and caused delayed O2 production, which implies a strong effect of daylength on the net O2 export from mats that host competitive photosynthetic and chemosynthetic communities. We assessed this prediction by measuring the net O2 production in these mats during different daylengths simulated in the laboratory under low O2 conditions (Figs. 2a and 3f, and Extended Data Fig. 7). For daylengths of <12 hours, no O2 was produced and the mats were a net sink for O2. For a daylength of 16 hours (that is, late Archean) and longer, a net diel O2 export occurred, with 21 hour (late Proterozoic) and 24 hour daylengths exporting two and three times, respectively, more O2 than 16 hour one.
Although similar competitive effects have been observed in other extant mat systems26, the applicability of these analogues to Precambrian mats is uncertain. However, several other mechanisms also result in a delayed O2 production due to a dramatic variation of the redox conditions in microbial mats within diel timescales. Some microbial groups are equipped with mechanisms to regulate or delay the onset of certain metabolic processes20,27,28,29. Similar to the MIS mat, the implementation of a delayed recovery of OP after exposure to sulfide27 in our modelled mat showed that GPP and Corg burial decreased sharply with decreasing daylength (Extended Data Fig. 8). Overall, the combined effect of mass transfer limitation, metabolic regulation and whole-community interactions in living microbial mats strengthens the dependency of O2 export and Corg burial on increasing daylength (Fig. 2).
The regulation of Corg burial by daylength and by the corresponding diel O2 dynamics is conceptually consistent with empirical observations in modern sediments, in which the long-term burial efficiency decreases with the exposure time to O2 (ref. 14). As longer days export more O2, the ratio between Raero and Ranaero decreases (Extended Data Fig. 3), the average diel O2 penetration depth decreases (Extended Data Fig. 2b) and the non-photosynthetic layers of mats are exposed to O2 for a shorter fraction of the day (Extended Data Fig. 2c). This suggests that daylength also promotes long-term Corg burial independent of the effect of daylength on the dynamic response of respiratory processes with specific metabolic traits. Additionally, mat accretion rates must be accounted for because they shape the residence time of Corg in the oxic zone. Modern mats reach stunning accretion rates30 (for example, 0.5–5 mm yr−1), similar to estimates from ancient microbialites31 (0.5–15 mm yr−1). Given that longer days decreased the diel local O2 availability and enhanced the burial efficiency, the accretion rate would have increased, thereby possibly establishing a positive feedback effect on long-term burial. This is because both regulatory factors result in shorter exposure times of benthic Corg to O2, but on different timescales. Thus, the daylength effect on net productivity and long-term burial could possibly be more pronounced for growing mats than for our modelled mats with a stagnant biomass.
Spinning down to oxygenation
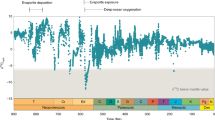
The fundamental effect of changing planetary rotation rate on benthic export fluxes would have applied for most of Earth’s photosynthetic history until the end of the ‘matworld’32. Quantitative assessment requires parameterizing global benthic Corg burial along with Earth’s rotation rate, which decreased over Earth’s history as inferred from geological proxies33,34 and models10,35. A precise reconstruction of the rotation rate is currently beyond reach owing to uncertainties in the strengths of tidal friction, which includes effects from oceanic33,36, atmospheric10,37 and solid Earth tides35. Although there is no consensus on the exact pattern, the rate of oceanic tidal dissipation normalized to the strength of the astronomical forcing must have been lower than modern rates for long stretches of Earth’s history because the current rate implies an Earth–Moon collision at ~1.5 Ga, for which there is no evidence36. Recent models that consider the effect of changes in the continental configuration on tidal dissipation rates suggest that Earth’s rotational deceleration was lowest in the mid-Proterozoic33. Another long-standing hypothesis even predicts a period with a stable rotation rate in the Proterozoic due to a resonant atmospheric thermal tide10,37. The dissipation of oceanic and solid Earth tides cause rotational deceleration that is counteracted by atmospheric thermal tides, which depend on daylength37. This hypothesized period of stability and the subsequent marked increase in daylength coincide with the boring billion years of O2 stasis and the NOE, respectively. The remarkable correlation between the patterns of Earth’s oxygenation and rotation rate (Fig. 4) invites a quantitative evaluation of the potential mechanistic link between daylength and oxygenation.
a, Estimates for modelled and empirical values for daylength as derived from various studies10,35,37,59, illustrate that daylength might have been nearly constant in the Proterozoic. This coincides with the boring billion6, and the Earth escaped the ‘resonance lock’ in the timeframe of the NOE. b, Estimates of the quasi-steady states of the global reservoir of \(p{{\rm{O}}_2}\) as a response to changes in coastal benthic and terrestrial Corg burial fluxes driven by changes in daylength. The proposed daylength effect on microbial mat fluxes was calculated based on the Earth rotation model by Bartlett and Stevenson10 (solid line in a). daylength can account for a substantial portion of the \(p{{\rm{O}}_2}\) changes associated with the GOE and NOE, which lead up to the POE. The oxygenation scheme (grey area) is shown for comparison and is adapted from Alcott et al.6. Fluxes are derived from our modelled scenarios, which represent systems that include Raero and Ranaero and either exclusive oxygenic or partitioned OP and AP (scenarios ‘OP SRB’ and ‘OP SRB + AP’). Scenarios with metabolic delay mechanisms or a steep dependency on the burial of O2 were excluded because these yielded oscillations of \(p{{\rm{O}}_2}\) between 0 and 1 even in the boring billion years. Shaded areas represent the range of daylength-driven \(p{{\rm{O}}_2}\) change based on a 1.5–3.7% coverage of the modern oceanic area by benthic coastal mats (which corresponds to 20–50% of the global marine Corg burial during the mid-Proterozoic) and a coverage of 5% of the continental area by terrestrial mats. The corresponding global O2 sinks were parameterized for a reference \(p{{\rm{O}}_2}\) of 0.1 and 0.01 in the mid-Proterozoic60. For terrestrial mats we assumed a direct interaction of 95% of their buried Corg with \(p{{\rm{O}}_2}\) by erosional weathering, which represents a negative feedback effect of daylength-driven Corg burial on \(p{{\rm{O}}_2}\).
We extended our estimates of diel benthic Corg burial to global scales over Earth’s history. Considerations of Corg fluxes on planetary and geological scales require several uncertain assumptions, and thus our estimates represent a possible range of the effect of rotation rate on \(p{{\rm{O}}_2}\). To quantitatively implement Earth’s rotational deceleration, we used a recent model10, which predicts deceleration before 2.2 Ga followed by resonant stability during the mid-Proterozoic until ~650 Ma, and subsequent return to deceleration towards the modern 24 h daylength. To model the quasi-steady-state evolution of global \(p{{\rm{O}}_2}\), we considered that daylength-driven changes in global Corg burial and solute export fluxes interacted with sinks of O2 beyond the benthic domain, namely, atmospheric reduction by metamorphism- and volcanism-derived gases and erosional weathering (Extended Data Fig. 1). As the relative contributions of benthic and pelagic to total marine Corg burial are highly uncertain38,39,40,41,42, we expressed the benthic term as a fraction of total marine burial, assumed to be at modern levels. Beyond the marine realm, we considered the possible effect of terrestrial mats in the Precambrian43. As we expect long-term burial rates to be substantially lower than the diel Corg burial14, we implemented a weathering-based negative feedback effect between \(p{{\rm{O}}_2}\) and terrestrial diel, and implicitly on long-term, Corg burial (Extended Data Figs. 1 and 9). This analysis showed that daylength-driven changes in Corg burial could account for the offset between pre- and post-GOE O2 levels without having to assume any changes in atmospheric reductant fluxes or GPP (Fig. 4b). Mat scenarios with no net Corg burial in the pre-GOE Archean (18 h daylength) support 50% of the global marine Corg burial in the mid-Proterozoic (21 h daylength), but only occupy 3.7% of the modern oceanic area (see Methods). For the modern continental arrangement, this mat coverage is comfortably less than the neritic zone (7.5%)44, the primary habitat for benthic photosynthesis. The daylength effect implies an increase up to ~0.28\(p{{\rm{O}}_2}\) at around 0.55 Ga (Fig. 4b), consistent with an early NOE and a later Palaeozoic oxidation event (POE), possibly connected to the Great Ordovician Biodiversity Event, at ~0.4 Ga (refs 2,45).
We suggest that changes in daylength rebalance remineralization. Positive carbon isotope excursions associated with oxygenation events are interpreted as signals of increased Corg burial, caused by increasing GPP or decreasing remineralization efficiency46,47,48. How these parameters imprint on the isotope record in the case of microbial mats is uncertain due to mass transfer limitation of dissolved inorganic carbon supply and the resultant negligible isotope fractionation observed in modern mats49,50,51. Yet, the daylength-driven enhancement of O2 export and Corg burial is consistent with proxies for weathering and total organic carbon contents in the record (Extended Data Fig. 9). Despite the predicted increase in weathering, we have not included the response of phosphorus fluxes to increasing \(p{{\rm{O}}_2}\). Increased weathering would have further boosted the global primary production7,52—but probably only transiently as a pulse, followed by a return to the previous quasi-steady state \(p{{\rm{O}}_2}\) (refs 3,6) for a given daylength. Although nutrient supply and the corresponding GPP determine the absolute range of \(p{{\rm{O}}_2}\) levels, daylength effects on benthic burial could have shaped the overall oxygenation pattern before the POE (Fig. 4b).
Overall, we show that increasing daylength is a monotonic driver of net productivity pervasive across all ranges of metabolic parameters (Fig. 2). Relatively abrupt changes in daylength, as caused by escape or entry into resonance locking, could therefore be among the triggers for the global oxidation events. In this respect, our proposed mechanism is similar to that of other abrupt events that cause imbalances in the global \(p{{\rm{O}}_2}\) budget, such as plate tectonics (including supercontinent formation)5,53,54,55 or new prospects for productivity in the oceans and on land4,45,56. As Earth’s rotation rate, governed by planetary physics, does not share any assumptions with these geological or biological triggers, the daylength effect operates in parallel to these other Earth-bound mechanisms. Even if we assume a more gradual decrease in rotation rate, our study clearly suggests that net productivity would have increased. Previous studies show that gradual changes in sources and sinks of O2 can cause relatively abrupt shifts in \(p{{\rm{O}}_2}\), such as those due to H2 escape, insolation, continental phosphorus supply, continental growth or volcanic reductant input6,23,47,57,58. The exact magnitude of the daylength effect remains uncertain as it relies on several assumptions, particularly the relationship between diel and long-term burial efficiency and the detailed pattern of rotational deceleration. Further, we have not considered the effects of changes in the limiting factors of gross productivity, such as phosphorus, insolation or strength of O2 sinks, other than daylength-related increases in terrestrial Corg weathering. Yet, the peculiarity of daylength-driven increases in benthic net productivity is that no such changes are required to produce substantial changes in \(p{{\rm{O}}_2}\). Thus, the dynamics of the Earth–Moon system possibly had major impacts on global O2 levels during critical turning points of Earth’s biogeochemical evolution towards a profusely oxic world.
Methods
Modelling microbenthic O2 export
We explored how microbial processes and export fluxes of their metabolic substrates and products from ancient benthic photosynthetic ecosystems were influenced by daylength, environmental conditions and various regulatory mechanisms of photosynthetic production and respiration using an in silico microbenthic model. Model scenarios were constructed and simulated using MicroBenthos software12. MicroBenthos model definitions and parameters for the described scenarios are provided with this article. The software and usage instructions are available at https://microbenthos.readthedocs.io.
The modelling framework is an adaptation of de Wit et al.61. Briefly, benthic systems are constructed as a diffusive–reactive system in a 1D computational domain, with discrete cells used to represent the spatial distribution of the state and parameter variables. While the study by de Wit et al.61 focused on biomass growth running over long simulation times, our interest was to study the dynamics of process rates and solute fluxes over diel timescales. Therefore, we set a fixed biomass for the microbial groups, added a water subdomain on top of the sediment as a diffusive boundary layer and ran simulations until a diel steady state was reached (5 days). Our model domain used 5 µm cells, with an 8 mm sedimentary subdomain and 1 mm diffusive boundary layer of water on top. O2 and sulfide concentrations were the state variables that we solved for. Photosynthetically active radiation (PAR) was expressed as a percent of the maximum intensity at the diel zenith, and followed a cosinusoidal pattern similar to that of diel insolation dynamics.
Raero and SOX were formulated to occur throughout the sediment. Microbial groups (cyanobacteria and SRB) were represented as biomass distributions in the sediment subdomain, and biomass-dependent metabolism kinetics were expressed as multiplications of the response functions of salient environmental and state variables. Coupled partial differential equations of the state variables (O2 and H2S) were composed from the reaction terms that accounted for sediment porosity and were solved with finite-volume numerical approximations62.
Our in silico mat allowed us to explore how diffusive mass transfer shapes the interplay between illumination dynamics, gross production and consumption rates, and diel O2 export. The effect of daylength was studied by varying the period of the illumination from 12 h to 24 h, the range of estimated daylengths over Earth’s history after the earliest estimates for the origin of OP63. We report the calculated average diel net export and process rates in units of mmol m−2 h−1 because the hour is the largest temporal unit unaffected by changes in the Earth’s rotation and thus allows for comparison across daylengths.
First, we explored the simplest case of O2 production, which is with light availability. Two microbial processes were considered: OP performed by cyanobacteria and Raero. The parameters for the biotic reactions were re-expressed as a biomass-specific maximal yield (Qmax). A fundamental assumption is that the photosynthesis rate is strictly correlated to the instantaneous photon flux:
where sat is a Michaelis–Menten function with KPAR = 15% and the cyanobacterial biomass with a log-normal distribution with a peak value of 12 mg cm−3 at 0.5 mm depth (Supplementary Video 1). The only source of O2 is OP, and the sinks are aerobic (sedimentary) respiration (Raero). For the production and consumption rates of Corg, we assumed a stoichiometry of:
with respect to O2 cycling rates, where CH2O refers to one Corg equivalent. Assuming that Corg is predominantly particulate, with negligible diffusional transport, diel Corg burial was thus calculated as:
where ∫OP and ∫Raero are the diel depth-integrated rates of O2 production and consumption and are equivalent to Corg production and consumption according to equation (2). Thus, diel burial can also be represented through the export flux of O2 at the top and bottom interfaces of the sedimentary domain:
which allowed us to assess the dynamic steady state of the diel model when the average diel depth-integrated rates equalled the export fluxes.
To calibrate the O2 productivity for unitless PAR intensities, we determined the Qmax that caused a maximum O2 export that corresponded to the median maximal flux from illuminated benthic photosynthetic systems13. A Qmax of 4.0022 mmol g−1 h−1 produced the target export flux of 5.76 mmol m−2 h−1 under a sedimentary respiration load of 0.1 mM h−1. Note that by calibrating the productivity to the maximum diel illumination, the model represents a ‘mean solar day’ of a given Earth year59. This allowed us to disentangle the effect of daylength from geological-scale changes in the insolation intensity, such as in the ‘faint young Sun’ paradigm reviewed thoroughly by Feulner23, or changes in the solar spectrum related to atmospheric composition64.
Next, we explored the effect of Ranaero on the daylength dependency of the process rates and export fluxes. We used the example of sulfate reduction performed by SRB with a log-normal biomass distribution with a peak value of 2 mg cm−3 (Supplementary Video 1). The Ranaero rate was either defined as a constant rate process for the scenario ‘OP SRB constant’ as:
or as an O2- and H2S-sensitive process as:
where inhibition is a function of the local H2S and O2 concentration (x) of the form:
when x < Kmax and 0 when x ≥ Kmax. Inhibition factors chosen for both scenarios with O2- and H2S-sensitive SRB (‘OP SRB’ and ‘OP SRB inhibited’) were 3 mM for \(K_{{\rm{max}},{\rm{H}}_2{\rm{S}}}\), 0.5 mM for \(K_{{\rm{half}},{\rm{H}}_2{\rm{S}}}\) and 1 µM for \(K_{{\rm{half}},{\rm{O}}_2}\). \(K_{{\rm{max}},{\rm{O}}_2}\) was 0.8 mM for the scenario with a moderate inhibition, ‘OP SRB’, and 0.3 mM for the scenario with a strong inhibition, ‘OP SRB inhibited’.
SOX was formulated as:
where k = 351 l mol−1 h−1 (ref. 65). For Ranaero we assumed the stoichiometry:
and therefore calculated diel burial as:
where ∫Ranaero is the depth-integrated rate of sulfide production by SRB. The export flux of O2 is shaped by mat-intrinsic rates of Raero, OP and SOX according to:
where ∫SOX is the depth-integrated rate of O2 consumption by SOX. Assuming the complete oxidation of H2S to sulfate, H2S export can be formulated as:
According to equations (10)–(12), diel burial can thus be represented as:
This illustrates that the control of diel burial is related to the export of O2 and the reduced product of Ranaero (such as H2S), as the former is an equivalent source and the latter an equivalent sink of Corg within the mat. This means that an increase in O2 export would not result in an increase of burial if H2S export increased proportionally in terms of Corg equivalents.
To calibrate the productivity for SRB scenarios, we determined values of Qmax for OP and SRB, which yielded the target maximum export flux of 5.76 mmol m−2 h−1 at 24 h under 250 µM O2 boundary conditions and negligible burial fluxes at 18 h under anoxic boundary conditions, that is, in pre-GOE conditions (see Supplementary Data 1 for model parameters).
We tested the sensitivity of diel burial to O2 concentration in the water column (0–250 µM) for all three SRB scenarios. For the least O2-sensitive scenario (‘OP SRB’), we also tested sensitivity of burial to gross productivity by varying the maximal photosynthetic yield Qmax over the range 0.5–10 mmol g−1 h−1. Note that the variation in Qmax can be considered equivalent to variations in other factors that influence gross production, such as nutrient supply and irradiance levels.
We then explored the effect of AP and reductant supply to the mat. Reductants that served as electron donors for AP were available in Precambrian phototrophic habitats, and supported diverse forms of photosynthesis even after the GOE and the evolution of OP66,67,68. Although the extent and ecological niches of AP and OP over Earth’s history remain unclear, AP and OP probably co-existed, with spatially and temporally variable partitioning of the total GPP between them69. Analogous to modern systems, the partitioning probably depended on the limiting factors of both metabolisms, such as light and nutrients, with AP additionally limited by electron donor supply, and on the onset of novel evolutionary avenues or geochemical transitions that facilitated shifts in the outcome of competition.
We implemented this concept by adapting the ‘OP SRB’ scenario to include metabolic flexibility in the modelled photosynthetic community, analogous to cyanobacteria that can partition harvested light energy towards OP and sulfide-driven AP (Extended Data Fig. 4). Transitions between photosynthetic modes are based on the local sulfide and light availability with the rate of OP regulated by the rate of AP20. In this ‘OP SRB + AP’ scenario, the modelled cyanobacteria produced O2 according to:
where normsat is a normalized Michaelis–Menten function with a value of 1 for H2S ≥ H2Sthr, where H2Sthr is the threshold sulfide level. Conversely,
The metabolic behavior is such that OP occurs only below H2Sthr, which is light dependent. Below this threshold level, the harvested light energy is partitioned towards AP and OP, with a higher affinity for AP. Above the threshold, only AP occurs and OP is suppressed. The resulting metabolic response is that the sum of OP and AP follows the form of equation (1), whereas Ranaero, Raero, SOX and other processes work as in previous scenarios. The interplay of illumination, mat processes and the resultant depth-resolved dynamics of O2 and H2S under this scenario can be seen in the Supplementary Video 1. Assuming sulfate as the product of AP according to:
diel burial in this scenario was calculated as
where ∫AP is the depth-integrated rate of CO2 fixation by AP. As in the other scenarios, diel burial can alternatively be calculated according to equation (13). Values of Qmax for OP and Ranaero were adjusted to produce the same maximum O2 export flux at 24 h (O2 boundary at 250 µM) and diel Corg flux at 21 h (25 µM O2 boundary) as in ‘OP SRB’. This allowed us to establish directly comparable upscaling calculations for global \(p{{\rm{O}}_2}\) at the same areal coverage and diel burial by ‘OP SRB’ and ‘OP SRB + AP’ mats (see below).
Besides the effect of daylength, we also studied the effect of the availability of O2 and H2S as electron donors from the water column on the scenario with AP. Water column O2 levels from 0 to 250 µM and H2S levels from 0 to 20 µM were tested.
Photosynthetic inhibitory mechanisms were also tested by modelling cyanobacteria that exhibit a 30 min recovery time of OP after the local sulfide levels fall below a certain level27.
Microsensor measurements
We sampled cores from cyanobacterial mats that formed in the MIS (Michigan, United States) in October 2015, May 2016 and June 2016. Cores and bottom water sampled from above the mats were transported to the lab in Ann Arbor. Cores were either used directly or kept on the seasonal day–night light cycle at 8 °C until the measurements were made. During the measurements in the cores, a circular flow of the water column above the mat surface was adjusted using peristaltic pumps connected to an external temperature-controlled reservoir of bottom water. Water column O2 and sulfide concentrations were adjusted in this separate reservoir of bottom water using N2, air and neutralized Na2S solution. After adjustment, the reservoir was covered with paraffin oil to prevent gas exchange and maintain constant conditions in the water column. The total sulfide concentration in the water column was regularly checked by subsampling and colorimetric determination according to Cline70. Both the water column reservoir and the core were kept at 10 °C during the measurements.
Illumination was provided by a broadband halogen lamp (Schott KL-1500). In situ spectral light quality was simulated using optical filter foils (Roscolux 375, Rosco Laboratories). The incident irradiance at the surface of the mat was determined with a submerged cosine-corrected quantum sensor connected to a LI-250A light meter (both LI-COR Biosciences GmbH). O2, pH and H2S microsensors with a tip diameter of 10–50 µm and a response time of <1 s were built, calibrated and employed as previously described71,72,73. The microsensor tips were always separated by <500 µm during simultaneous O2, pH and H2S measurements.
The volumetric rates of gross OP were estimated based on the dynamics of O2 concentration after a light–dark shift, as described previously74. Analogously, the volumetric rates of gross AP were calculated from the increase of H2S concentration and pH directly after a light–dark transition (that is, light-driven Stot consumption rates), as previously described26,27,75. Net rates and fluxes were calculated from profiles based on Fick’s laws using diffusion coefficients for O2 and sulfide corrected for temperature and salinity.
From diel export to global oxygenation
We considered that changes in diel O2 and H2S export rates, the difference of which is equivalent to diel benthic Corg burial, interact with global redox controls beyond the mat domain (Extended Data Fig. 1). Namely, we considered atmospheric reduction by volcanism- and metamorphism-derived gases (atmR) and weathering60. Although we assumed a constant flux of reductant (vR) available for atmospheric reduction, weathering is dependent on \(p{{\rm{O}}_2}\) and thus strongly tempers the accumulation of O2 in the atmosphere. As a recent computational Earth rotation model10 predicts a stabilized daylength in the mid-Proterozoic, we started our global calculations by assuming a \(p{{\rm{O}}_2}\) of 0.01–0.1 at the predicted stable 21 h. This \(p{{\rm{O}}_2}\) is the result of the interaction of global sources and sinks (illustrated in Extended Data Fig. 1) according to a simplified version of the formulations (for example, COPSE60,76) of the global O2 budget. Considering an evolution of steady states of \(p{{\rm{O}}_2}\) throughout Earth’s history, we described the \(p{{\rm{O}}_2}\) evolution at a given age as:
which provides the steady state \(p{{\rm{O}}_2}\) as:
where pB is the global burial flux associated with marine pelagic production, mB is total mat burial flux, vR is the flux of volcanic reductant, tB is the burial flux by terrestrial mats, and uB is an aggregate flux term that captures uplift forcing, the global Corg reservoir and a weathering constant60.
Changes in any of the global fluxes would therefore result in new steady state \(p{{\rm{O}}_2}\). For the total marine burial (pelagic pB and coastal benthic mB – tB) we assumed modern values throughout the Precambrian60. Beyond the marine realm, we considered the possible existence of terrestrial mats43, which would have further boosted global GPP and Corg burial, but would have also been more susceptible to weathering (tB in Extended Data Fig. 1). For these terrestrial mats, we assumed the same regulation mechanisms of export fluxes as those for coastal benthic mats and considered that AP might have been driven by reductant supplied from Ranaero, but we did not explore the effects of external reductant availability for terrestrial mats. Using our diel mat model output for tB at 21 h daylength and for 5% continental coverage, we then calculated vR for the two \(p{{\rm{O}}_2}\) levels assuming the values for uB from Daines et al.60.
The Earth rotation rate model10 predicts monotonic deceleration before 2.2 Ga followed by a stable daylength of ~21 h due to the atmospheric thermal tide resonance until ~650 Ma, with a subsequent increase towards the modern 24 h daylength10. Starting at 21 h at 2 Gyr, we estimated the effect of the daylength-driven change in the Corg burial from benthic and terrestrial mats, the output of our diel mat model, on \(p{{\rm{O}}_2}\) levels both backwards and forwards in time. The modulation of \(p{{\rm{O}}_2}\) naturally depends on the steepness of the dependence of mat Corg burial on daylength (Fig. 2). However, we limited our analysis to the effect of mats with metabolic regulation from the moderately daylength-sensitive scenarios ‘OP SRB’ and ‘OP SRB + AP’. Scenarios with a steeper dependency of burial on daylength yielded oscillations of \(p{{\rm{O}}_2}\) beyond PAL even due to the minimal variations in daylength within the resonant-locked phase in the mid-Proterozoic10 and therefore had to be excluded from analysis of \(p{{\rm{O}}_2}\) evolution. We additionally included negative feedback effects of increasing \(p{{\rm{O}}_2}\) on the mat Corg burial by dynamically adjusting the O2 boundary conditions in each time step according to the \(p{{\rm{O}}_2}\) calculated from the previous step. To then calculate the quasisteady-state \(p{{\rm{O}}_2}\) level based on changes in the benthic and terrestrial burial, the actual coverage and thus partitioning between the pelagic and benthic burial have to be considered. Reliable estimates for this partitioning are lacking. The presence of pelagic cyanobacteria has persisted since 1.1 Ga (ref. 38). Yet, cyanobacteria in the palaeontological record are primarily benthic, with sparse evidence for pelagic forms39,40,41. Hypotheses for the limited pelagic productivity during the Proterozoic range from an inaccessible, toxic or damaging photic zone to late evolution of a planktonic lifestyle21,66,77. Alternatively, burial of the Corg produced by pelagic cyanobacteria might have been hindered by the small cell sizes, low sinking rates and resultant efficient remineralization within the water column42,78. The preservation of Corg by benthic microbial mats is as uncertain, especially when eroded—on land or in the oceans. In our calculations we considered the terrestrial erosional weathering explicitly (tB in Extended Data Fig. 1), which introduces a strong negative feedback effect on \(p{{\rm{O}}_2}\). This implicitly describes a substantial loss in translation of the diel Corg to long-term burial that we, however, did not explicitly account for. For marine benthic mats we argue that a more direct link between the diel and long-term burial of Corg is plausible given that mats can reach substantial thicknesses when undisturbed (for example, in Solar Lake the thickness is >1 m) (ref. 79) and that mats have a similar remineralization fate as pelagic Corg export when eroded. To address these uncertainties, we considered benthic burial as between 20 and 50% of the total marine burial at 21 h daylength. Note that this partitioning must shift during our simulations over Earth history because benthic (and terrestrial) burial is daylength dependent, whereas we took an ‘all is constant’ approach for pelagic burial and the fraction of weathered diel Corg, as we expected a negligible effect of molecular diffusion and thus daylength on these fluxes.
Data availability
The datasets generated and analysed are available at https://doi.org/10.17617/3.66, and in the supplementary files with this paper. Source data are provided with this paper.
Code availability
The open source MicroBenthos framework (v0.15) was used to model the presented results. Documentation is available at https://microbenthos.readthedocs.io and the code at https://github.com/achennu/microbenthos.git. Model definition files specific to this study are included in the supplementary files. Software to simulate the daylength history was obtained from https://github.com/bencbartlett/lengthofday.
Change history
29 October 2021
In the version of this article initially published online, the following metadata was omitted and has now been included: “Open access funding provided by Max Planck Institute for Marine Microbiology (2)”.
References
Lyons, T. W., Reinhard, C. T. & Planavsky, N. J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014).
Och, L. M. & Shields-Zhou, G. A. The Neoproterozoic oxygenation event: environmental perturbations and biogeochemical cycling. Earth Sci. Rev. 110, 26–57 (2012).
Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014).
Brocks, J. J. et al. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 548, 578–581 (2017).
Williams, J. J., Mills, B. J. & Lenton, T. M. A tectonically driven Ediacaran oxygenation event. Nat. Commun. 10, 2690 (2019).
Alcott, L. J., Mills, B. J. W. & Poulton, S. W. Stepwise Earth oxygenation is an inherent property of global biogeochemical cycling. Science 366, 1333–1337 (2019).
Reinhard, C. T. et al. Evolution of the global phosphorus cycle. Nature 541, 386–389 (2017).
Lambeck, K. The Earth’s Variable Rotation: Geophysical Causes and Consequences (Cambridge Univ. Press, 1980).
Munk, W. H. & MacDonald, G. J. F. The Rotation of the Earth: A Geophysical Discussion (Cambridge Univ. Press, 1960).
Bartlett, B. C. & Stevenson, D. J. Analysis of a Precambrian resonance-stabilized day length. Geophys. Res. Lett. 43, 5716–5724 (2016).
Ćuk, M. & Stewart, S. T. Making the Moon from a fast-spinning Earth: a giant impact followed by resonant despinning. Science 338, 1047–1052 (2012).
Chennu, A. MicroBenthos: a modeling framework for microbial benthic ecology. J. Open Source Softw. 3, 674 (2018).
Lalonde, S. V. & Konhauser, K. O. Benthic perspective on Earth’s oldest evidence for oxygenic photosynthesis. Proc. Natl Acad. Sci. USA 112, 995–1000 (2015).
Sarmiento, J. L. & Gruber, N. in Ocean Biogeochemical Dynamics (eds Sarmiento, J. L. & Gruber, N.) 227–269 (Princeton Univ. Press, 2006).
Shen, Y. & Buick, R. The antiquity of microbial sulfate reduction. Earth Sci. Rev. 64, 243–272 (2014).
Reinhard, C. T. et al. Proterozoic ocean redox and biogeochemical stasis. Proc. Natl Acad. Sci. USA 110, 5357–5362 (2013).
Fründ, C. & Cohen, Y. Diurnal cycles of sulfate reduction under oxic conditions in cyanobacterial mats. Appl. Environ. Microbiol. 58, 70–77 (1992).
Canfield, D. E. & Des Marais, D. J. Aerobic sulfate reduction in microbial mats. Science 251, 1471–1473 (1991).
Canfield, D. E. & Des Marais, D. J. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim. Cosmochim. Acta 57, 3971–3984 (1993).
Klatt, J. M. et al. Anoxygenic photosynthesis controls oxygenic photosynthesis in a cyanobacterium from a sulfidic spring. Appl. Environ. Microbiol. 81, 2025–2031 (2015).
Sánchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 5, 17418 (2015).
Valentine, J. W. & Moores, E. M. Plate-tectonic regulation of faunal diversity and sea level: a model. Nature 228, 657–659 (1970).
Feulner, G. The faint young Sun problem. Rev. Geophys. 50, RG2006 (2012).
Olson, S. L., Jansen, M. & Abbot, D. S. Oceanographic considerations for exoplanet life detection. Astrophys. J. 895, 19 (2020).
Voorhies, A. A. et al. Cyanobacterial life at low O2: community genomics and function reveal metabolic versatility and extremely low diversity in a Great Lakes sinkhole mat. Geobiology 10, 250–267 (2012).
Klatt, J. M. et al. Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy. ISME J. 10, 921–933 (2016).
Hamilton, T. L., Klatt, J. M., de Beer, D. & Macalady, J. L. Cyanobacterial photosynthesis under sulfidic conditions: insights from the isolate Leptolyngbya sp. strain hensonii. ISME J. 12, 568–584 (2018).
Oren, A. & Padan, E. Induction of anaerobic, photoautotrophic growth in the cyanobacterium Oscillatoria limnetica. J. Bacteriol. 133, 558–563 (1978).
Garcia-Pichel, F. & Castenholz, R. W. Comparative anoxygenic photosynthetic capacity in 7 strains of a thermophilic cyanobacterium. Arch. Microbiol. 153, 344–351 (1990).
Krumbein, W. E., Cohen, Y. & Shilo, M. Solar Lake (Sinai). 4. Stromatolitic cyanobacterial mats. Limnol. Oceanogr. 22, 635–656 (1977).
Lanier, W. P. Approximate growth rates of early Proterozoic microstromatolites as deduced by biomass productivity. Palaios 1, 525–542 (1986).
Lenton, T. M. & Daines, S. J. Matworld—the biogeochemical effects of early life on land. New Phytol. 215, 531–537 (2016).
Meyers, S. R. & Malinverno, A. Proterozoic Milankovitch cycles and the history of the solar system. Proc. Natl Acad. Sci. USA 115, 6363–6368 (2018).
Williams, G. E. Late Precambrian tidal rhythmites in South Australia and the history of the Earth’s rotation. J. Geol. Soc. 146, 97–111 (1989).
Ross, M. N. & Schubert, G. Evolution of the lunar orbit with temperature- and frequency-dependent dissipation. J. Geophys. Res. 94, 9533 (1989).
Bills, B. G. & Ray, R. D. Lunar orbital evolution: a synthesis of recent results. Geophys. Res. Lett. 26, 3045–3048 (1999).
Zahnle, K. & Walker, J. C. A constant daylength during the Precambrian era? Precambrian Res. 37, 95–105 (1987).
Gueneli, N. et al. 1.1-billion-year-old porphyrins establish a marine ecosystem dominated by bacterial primary producers. Proc. Natl Acad. Sci. USA 115, E6978–E6986 (2018).
Sergeev, V. N., Gerasimenko, L. M. & Zavarzin, G. A. The Proterozoic history and present state of cyanobacteria. Microbiology 71, 623–637 (2002).
Demoulin, C. F. et al. Cyanobacteria evolution: insight from the fossil record. Free Radic. Biol. Med. 140, 206–223 (2019).
Dodd, M. S., Papineau, D., Pirajno, F., Wan, Y. & Karhu, J. A. Minimal biomass deposition in banded iron formations inferred from organic matter and clay relationships. Nat. Commun. 10, 5022 (2019).
Butterfield, N. J. Macroevolutionary turnover through the Ediacaran transition: ecological and biogeochemical implications. Geol. Soc. Spec. Publ. 326, 55–66 (2009).
Finke, N. et al. Mesophilic microorganisms build terrestrial mats analogous to Precambrian microbial jungles. Nat. Commun. 10, 4323 (2019).
Cahoon, L. B. in Oceanography and Marine Biology, An Annual Review (eds Ansell, A. et al.) 47–86 (Aberdeen Univ. Press, 1999).
Krause, A. J. et al. Stepwise oxygenation of the Paleozoic atmosphere. Nat. Commun. 9, 4081 (2018).
Derry, L. A., Kaufman, A. J. & Jacobsen, S. B. Sedimentary cycling and environmental change in the Late Proterozoic: evidence from stable and radiogenic isotopes. Geochim. Cosmochim. Acta 56, 1317–1329 (1992).
Hayes, J. M. & Waldbauer, J. R. The carbon cycle and associated redox processes through time. Philos. Trans. R. Soc. Lond. B. 361, 931–950 (2006).
Des Marais, D. J., Strauss, H., Summons, R. E. & Hayes, J. M. Carbon isotope evidence for the stepwise oxidation of the Proterozoic environment. Nature 359, 605–609 (1992).
Wieland, A., Pape, T., Möbius, J., Klock, J. H. & Michaelis, W. Carbon pools and isotopic trends in a hypersaline cyanobacterial mat. Geobiology 6, 171–186 (2008).
Schidlowski, M., Gorzawski, H. & Dor, I. Carbon isotope variations in a solar pond microbial mat: role of environmental gradients as steering variables. Geochim. Cosmochim. Acta 58, 2289–2298 (1994).
Des Marais, D. J. & Canfield, D. E. in Microbial Mats (eds Stal, L. J. & Caumette, P.) 289–298 (Springer, 1994).
Canfield, D. E., Poulton, S. W. & Narbonne, G. M. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science 315, 92–95 (2007).
Kump, L. R. & Barley, M. E. Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448, 1033–1036 (2007).
Lee, C.-T. A. et al. Two-step rise of atmospheric oxygen linked to the growth of continents. Nat. Geosci. 9, 417–424 (2016).
Campbell, I. H. & Allen, C. M. Formation of supercontinents linked to increases in atmospheric oxygen. Nat. Geosci. 1, 554–558 (2008).
Butterfield, N. J. Early evolution of the Eukaryota. Palaeontology 58, 5–17 (2015).
Zahnle, K. & Catling, D. Waiting for O2. Spec. Pap. Geol. Soc. Am. 504, 37–48 (2014).
Mills, B., Lenton, T. M. & Watson, A. J. Proterozoic oxygen rise linked to shifting balance between seafloor and terrestrial weathering. Proc. Natl Acad. Sci. USA 111, 9073–9078 (2014).
Williams, G. E. Geological constraints on the Precambrian history of Earth’s rotation and the Moon’s orbit. Rev. Geophys. 38, 37–59 (2000).
Daines, S. J., Mills, B. J. W. & Lenton, T. M. Atmospheric oxygen regulation at low Proterozoic levels by incomplete oxidative weathering of sedimentary organic carbon. Nat. Commun. 8, 14379 (2017).
de Wit, R., Ende, F. P. & Gemerden, H. Mathematical simulation of the interactions among cyanobacteria, purple sulfur bacteria and chemotrophic sulfur bacteria in microbial mat communities. FEMS Microbiol. Ecol. 17, 117–136 (1995).
Guyer, J. E., Wheeler, D. & Warren, J. A. FiPy: partial differential equations with Python. Comput. Sci. Eng. 11, 6–15 (2009).
Fischer, W. W., Hemp, J. & Johnson, J. E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 44, 647–683 (2016).
Arney, G. et al. The pale orange dot: the spectrum and habitability of hazy Archean Earth. Astrobiology 16, 873–899 (2016).
Luther, G. W. et al. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2, 62 (2011).
Johnston, D. T., Wolfe-Simon, F., Pearson, A. & Knoll, A. H. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth’s middle age. Proc. Natl Acad. Sci. USA 106, 16925–16929 (2009).
Jones, C., Nomosatryo, S., Crowe, S. A., Bjerrum, C. J. & Canfield, D. E. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology 43, 135–138 (2015).
Kharecha, P., Kasting, J. & Siefert, J. A coupled atmosphere–ecosystem model of the early Archean Earth. Geobiology 3, 53–76 (2005).
Ozaki, K., Thompson, K. J., Simister, R. L., Crowe, S. A. & Reinhard, C. T. Anoxygenic photosynthesis and the delayed oxygenation of Earth’s atmosphere. Nat. Commun. 10, 3026 (2019).
Cline, J. D. Oxygenation of hydrogen sulfide in seawater at constant salinity, temperature and pH. Environ. Sci. Technol. 3, 838–843 (1969).
Revsbech, N. P. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 34, 474–478 (1989).
Jeroschewski, P., Steuckart, C. & Kühl, M. An amperometric microsensor for the determination of H2S in aquatic environments. Anal. Chem. 68, 4351–4357 (1996).
de Beer, D., Schramm, A., Santegoeds, C. M. & Kühl, M. A nitrite microsensor for profiling environmental biofilms. Appl. Environ. Microbiol. 63, 973–977 (1997).
Revsbech, N. P. & Jørgensen, B. B. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: capabilities and limitations of the method. Limnol. Oceanogr. 28, 749–759 (1983).
Klatt, J. M., de Beer, D., Häusler, S. & Polerecky, L. Cyanobacteria in sulfidic spring microbial mats can perform oxygenic and anoxygenic photosynthesis simultaneously during an entire diurnal period. Front. Microbiol. 7, 1973 (2016).
Bergman, N. M., Lenton, T. M. & Watson, A. J. COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 304, 397–437 (2004).
Cockell, C. S. Ultraviolet radiation and the photobiology of Earth’s early oceans. Orig. Life Evol. Biosph. 30, 467–500 (2000).
Knoll, A. H., Summons, R. E., Waldbauer, J. R. & Zumberge, J. E. in Evolution of Primary Producers in the Sea (eds Falkowski, P. & Knoll, A.) 133–163 (Elsevier, 2007).
Krumbein, W. E. & Cohen, Y. Biogene, klastische und evaporitische Sedimentation in einem mesothermen monomiktischen ufernahen See (Golf von Aqaba). Geol. Rundsch. 63, 1035–1065 (1974).
Lasaga, A. C. & Ohmoto, H. The oxygen geochemical cycle: dynamics and stability. Geochim. Cosmochim. Acta 66, 361–381 (2002).
Nelson, D. C. & Castenholz, R. W. Light responses of Beggiatoa. Arch. Microbiol. 131, 146–155 (1982).
Biddanda, B. A., McMillan, A. C., Long, S. A., Snider, M. J. & Weinke, A. D. Seeking sunlight: rapid phototactic motility of filamentous mat-forming cyanobacteria optimize photosynthesis and enhance carbon burial in Lake Huron’s submerged sinkholes. Front. Microbiol. 6, 930 (2015).
Klatt, J. M. et al. Versatile cyanobacteria control the timing and extent of sulfide production in a Proterozoic analog microbial mat. ISME J. 14, 3024–3037 (2020).
Acknowledgements
We thank the technicians of the Microsensor Group for the sensor construction, V. Meyer (MPIMM) for advice during the measurements, D. de Beer (MPIMM), G. Druschel (IUPIU), H. Marchant (MPIMM), S. Grim (UofM) and S. Ruberg (NOAA GLERL) for discussions, and M. Medina, H. C. Tan, K. Olsen, O. Metcalf, K. Meyer, M. Powers and D. Smith from University of Michigan for their lab and logistical help. We are grateful for the support of R. Green, J. Bright, P. Hartmeyer, W. Lusardi, S. Gandulla and T. Smith from NOAA TBNMS. We thank B. C. Bartlett for making the Earth rotation simulation code available. This study was funded by NSF grants EAR1637066 and EAR1637093, NASA grant 80NSSC20K1135, the Max Planck Society and the University of Michigan Turner Fellowship.
Funding
Open access funding provided by Max Planck Institute for Marine Microbiology (2).
Author information
Authors and Affiliations
Contributions
J.M.K., B.K.A. and G.J.D. conceptualized the work. The methodology was provided by J.M.K. and A.C., the software by A.C., validation by J.M.K. and A.C., formal analysis by J.M.K. and A.C., investigation by J.M.K., resources by G.J.D., data curation by J.M.K. and A.C., original draft manuscript preparation by J.M.K., A.C. and G.J.D., draft review and editing by J.M.K., A.C., B.K.A., B.A.B. and G.J.D., visualization by J.M.K. and A.C., supervision by G.J.D. and funding acquisition by J.M.K., B.A.B. and G.J.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Geoscience thanks Lee Kump, Timothy Lyons and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rebecca Neely.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic of the global sinks and sources of O2 with net release vs uptake of reductant by mats.
The daylength-driven changes in Corg burial from benthic or terrestrial mats (mB; flux arrows not to scale) cause quasi steady-state transitions of global atmospheric pO2. Offsets in pO2 between such steady states are conceptualized here as aO. The diel mat processes (inset box) produce Corg burial fluxes (mB), which along with burial from the pelagic domain (pB) comprise the global O2 source. Both O2 (mO) and reductant (mR) export from mats are controlled by the interaction between mass transfer and mat-intrinsic process rates (oxygenic photosynthesis, OP; anoxygenic photosynthesis, AP; aerobic respiration, Raero; sulfate reduction, Ranaero; aerobic H2S oxidation, SOX), and hence are sensitive to daylength changes. For the global O2 sinks, we considered that some of the surplus O2 released from the terrestrial or marine realm was consumed directly in the atmosphere (atmR) by volcanism- and metamorphism-derived gases (vR)60. Surplus reductant released from mats (mR in (a)) will increase atmR. Surplus reductant consumed by mats (mR in (b)) will decrease atmR, and add to source strength mB. Thus, mat Corg burial mB is the sum of O2 export mO and reductant import mR, and also sensitive to daylength. Note that volcanic reductant fluxes (vR) are equal to pelagic Corg burial (pB) and the equivalent pelagic O2 export (pO) to illustrate that reductant uptake by mats influence the global availability of reductant. This influences the consumed fraction of pO by atmR. As a result, mB is equal to wO, that is the O2 that escapes reduction by atmR. The sink for wO is erosional weathering (WEATH), and the emergent pO2 for a reference weathering level is (wO/ (0.95 × tB + uB))2 76,80. uB, which implicitly describes the size of the global Corg reservoir, uplift forcing and a weathering constant, was chosen based on a mid-Proterozoic pO2 of 0.01 or 0.1 and was set constant over Earth age. To account for the direct erosion of terrestrial mats, WEATH was set to interact with 95% of terrestrial Corg burial rates (tB; a fraction of total mat burial mB). While this makes WEATH also sensitive to daylength and produces a buffering effect through increased weathering strength, atmospheric oxygenation aO still increases with daylength (Fig. 4).
Extended Data Fig. 2 Daylength-dependency of oxygen penetration depth, downward O2 flux, and diel fraction with oxic conditions below the photic zone in various benthic mat scenarios.
The total mat depth is 8 mm. a, Similar to the O2 export flux across the upper mat interface, the downward flux is modulated by daylength. Given the proximity of the mat to the bottom substrate, such as pyrite, which is prone to abiotic oxidation reactions, this downward O2 flux would increase pyrite weathering and sulfate release13. b, Despite the increase in fluxes, the diel oxygen penetration depth, measured as the depth which is exposed to at least 1µM O2, decreases with daylength. c, Similarly, the fraction of a day, during which deep zones of the mat (here 7 mm) are exposed to > 1µM O2 decreases with increasing daylength. Together, the factors in b & c represent a decrease of exposure time of deep Corg to O2, and are likely to have boosted burial efficiency with increasing daylength.
Extended Data Fig. 3 Daylength-dependency of mat processes and export fluxes explored for three scenarios with differently adapted sulfate reducers.
One scenario included anaerobic respiration that is not affected by changes in the O2 concentrations within the mat (OP SRB constant), ie. with constant anaerobic respiration across daylengths. The other two scenarios (OP SRB and OP SRB inhibited) implement, with variable inhibition degree, O2- and H2S-sensitivity of anaerobic respiration rates17,18,19. As increasing O2 in the water column ([O2]top) would have accompanied daylength increases over Earth’s history, we explored daylength-dependent activity rates and export fluxes for O2 boundary conditions 0 µM and 25 µM (pO2 = 0 and 0.1 PAL). a, O2 export consistently increases with daylength. b, Consequently, Raero decreases. In the scenario with Ranaero set constant, Raero is strongly enhanced under higher O2 level in the water column. c, Corg burial, which is shaped by GPP, Raero, Ranaero and diffusional mass transfer also increases with daylength. The increase of [O2]top reduces burial for the scenario with constant Ranaero due to the increase in Raero. Negative burial fluxes arise because model parameters were tuned such that burial approaches zero at 18 h (pre-GOE) for [O2]top of 0 µM. d, H2S export consistently increases with daylength. This implies that H2S export partially counteracts the effect of increased O2 export on burial compared to a purely aerobic scenario (Fig. 1). e, For O2-inhibited SRB, increasing water column O2 reduces anaerobic respiration rates. Consequently, Corg burial consistently increases with daylength but remains approximately independent of [O2]top in the scenario with moderate inhibition (OP SRB) and increases with [O2]top in the scenario with strong inhibition (OP SRB inhibited) f, The ratio between respiratory processes Raero and Ranaero decreases across scenarios and boundary conditions, indicating a rebalancing of reminerilization activity due to daylength change. Empirical long-term Corg burial rates are negatively correlated with this ratio and the exposure time of Corg to O214 (see Extended data figure 2).
Extended Data Fig. 4 Rates of cyanobacterial OP and AP depend on sulfide concentration and irradiance.
(Irradiance as percent of zenith value) For conversion of CO2 fixation rates into rates of photosynthetic O2 production and sulfide consumption, we assumed that OP follows 2 H2O + CO2 → O2 + Corg and AP follows H2S + 2 CO2 → SO42− + 2 Corg, respectively. Partitioning between OP (green lines) and AP (blue lines) in these cyanobacteria is regulated through light-dependent sulfide threshold levels20. Below the threshold level, OP and AP occur in concert such that their combined rate (OP + AP) is conserved. Above the threshold level, only AP occurs representing a higher affinity for AP. This type of partitioning represents a metabolic competition between OP and AP within the cyanobacteria. Notably, based on local light levels, AP would have to occur at a sufficiently high rate to deplete the local sulfide concentration below the threshold for OP to occur and produce O2.
Extended Data Fig. 5 Modeled temporal evolution of mat processes and export fluxes over 12 vs 24 h daylengths and and daylength-dependency of their diel averages explored for mat with AP.
a & b, Based on partitioning regulation between AP (dotted fill) and OP (Extended data figure 4), AP first has to consume local sulfide (dotted line), after which OP occurs at its maximum light-dependent rate. Total photosynthesis (∫zPAP+OP, grey fill) remains independent of daylength. c, The overall effect of daylength is that longer days export more O2 and bury more Corg. Note that the negative burial flux at 12 h arises because model parameters were tuned such that burial at 21 h is comparable to the scenario without AP (OP SRB in Extended data figure 3). d-k, As both reductant and O2 exposure of the mat have varied temporally and spatially, we explored the sensitivity of daylength-dependent changes in mat processes and fluxes across various [H2S]top and [O2]top boundary conditions. Burial increases with increasing [H2S]top and decreases with [O2]top. Yet, the effect of water column redox is negligible compared to the effect of daylength.
Extended Data Fig. 6 Dependency of the lag duration of SOB migration in MIS mats on irradiance and water column concentrations of O2 and H2S.
a, Net OP was calculated based on O2 microsensor depth profiling at three light intensities in one MIS mat sample. Mat-forming large sulfur oxidizing bacteria (SOB) are known to respond to light and migrate downwards upon sunrise81, while cyanobacteria are expected to migrate upwards82. However, similar to observations in the Frasassi Sulfidic Springs26,83, MIS-SOB shade cyanobacteria and inhibit OP and showed no direct photophobic response. Instead, simultaneous depth profiling with an H2S microsensor showed that migration only occurred if sulfide in the SOB layer was entirely depleted, such as after a sufficient duration of AP activity. Incident irradiance levels of 59 and 82 µmol photons m−2 s−1 were sufficiently high to allow for entire depletion of sulfide (solid and dashed lines in a) by AP in the cyanobacterial layer and to thereby limit sulfide supply to the SOB layer at the mat surface. The migration lag (~2.5 hours) after depletion of sulfide is indicated by the gray shaded area. Note that the duration of the lag is independent of light intensity. Light intensity of 51 µmol photons m−2 s−1 was insufficient for sulfide depletion (dotted lines) and consequently migration did not occurr. Thus, even though light had no direct effect on migration behavior, sufficient light intensity to sustain high rates of AP was necessary to deplete sulfide in order to trigger migration. b, The duration of the lag phase was monitored using an O2 and H2S microsensor under diverse water column conditions in two distinct mat samples. Irradiance during measurements was 73 and 103 µmol photons m−2 s−1 in mat 1 and mat 2, respectively. Data result from continuous profiling and involve an uncertainty of ~15-20 min due to the acquisition time of the profiles. Error bars and values in parenthesis in the legend represent the standard deviation of O2 and H2S concentration, respectively, averaged over three depths and 5-32 timepoints during the time series experiment (n=15-96 dependent on delay duration). Considering that increased sulfide supply from the water column extended the migration lag, migration might be induced via electron donor starvation of the SOB.
Extended Data Fig. 7 Microsensor depth profiles of O2 over simulated daylengths (12–52 h).
a-e, Dynamics of O2 export flux (solid lines) were calculated from the concentration depth profiles (color-coded maps) and used to estimate the diel period average (text in panel). Compiled export fluxes are shown in Fig. 3f. Light intensity during measurements is indicated by the top bar in µmol photons m2 s−1. Total sulfide concentration in the water column was <1 µM. Results highlight the substantial dependency of O2 export on daylength in MIS mats.
Extended Data Fig. 8 Simulations of benthic systems with activity delay mechanisms.
a-b, For sulfide-inhibited OP as observed in cyanobacteria isolated from Little Salt Spring, that only activate OP with a fixed delay of 30 min after local depletion of sulfide by AP27, the temporal evolution over a 12 h and 24 h diel period illustrates that this no-photosynthesis-phase between sulfide being depleted below 1 µM and OP being activated completely suppresses OP in days shorter than 20 h (panel e). The penalty on O2 export is high enough that cyanobacterial mats, despite the potential for OP, remain net sinks of O2 during illuminated periods. c, The delay introduces a steep dependency of diel export fluxes and burial on daylength. Note that negative burial fluxes arise because model parameters were tuned such that burial at 21 h is comparable to the scenario without AP (OP SRB in Extended data figure 2) and the AP scenario without delay (Extended data figure 5). d-k, The penalty of the lag on induction of OP is only overcome during longer days and in the presence of O2 in the water column ([O2]top). Mats can only net accumulate Corg, if OP is active. As the fraction of day during which OP can occur is strongly dependent on daylength, this scenario exhibits the steepest dependency of burial on daylength compared to all other scenarios.
Extended Data Fig. 9 Weathering and Corg burial rates over time and corresponding examples for proxies in the geological record.
Values for the total organic carbon (TOC) content in organic-rich sediments, the normalized seawater 87Sr/86Sr, and the average δ34S of sulfate were adapted from Och et al2, with permission from Elsevier. Increases in the latter two parameters indicate enhanced weathering fluxes. All rates were derived from our modeled scenario that include aerobic and anaerobic respiration and exclusive oxygenic photosynthesis. Shaded areas represent the range of rates dependent on 1.5–3.7% modern oceanic coverage by benthic coastal mats (corresponding to 20–50% of global marine Corg burial during the mid-Proterozoic) and a continental coverage of 5% by terrestrial mats. Changes in global coastal benthic and terrestrial Corg burial fluxes are driven by changes in daylength and are shaped by feedback effects of increasing pO2 (Fig. 4) on aerobic respiration. Pelagic burial, atmospheric reduction by volcanism- and metamorphism-derived gases and weathering were parameterized for a reference pO2 of 0.1 in the mid-Proterozoic60. The rate of atmospheric reduction was assumed to be constant and determined by the flux of reduced gases. In contrast, the rate of erosional weathering increases with daylength as it depends on pO2 and Corg burial by terrestrial mats.
Supplementary information
Supplementary Video 1
Visualization of the MicroBenthos model during a simulation. The sedimentary subdomain (8 mm) is shaded in brown, and the diffusive boundary layer (DBL, 1 mm) is shaded in cyan with the vertical axis as depth in mm. All profiles (except env.par) are unit normalized with the factor shown in the legends and the units shown on the bottom axis. The Microbes panel shows the distributions of the cyanobacteria (cyano) and sulfate-reducing bacteria (srb) populations, as well as the photosynthetically active radiation (PAR; env.par, top scale) distribution due to sediment attenuation and cyanobacterial photopigment absorption. The Processes panel shows all the generative/consumptive process terms for both O2 and sulfide, whereas the Sources panel shows the distribution of all processes combined. The Environment panel shows the profiles of O2 and sulfide concentrations resultant from numerical simulation at the simulated diel time point shown on the top right. The export flux of O2 and sulfide from the DBL to the water above is calculated based on the gradient of the concentration at the top of the DBL.
Supplementary Data 1
A ZIP archive of the MicroBenthos model definition files for all microbial mat scenarios used in this study. The definition are plain text files in YAML format, and can be used according to the instructions at http://microbenthos.readthedocs.io.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klatt, J.M., Chennu, A., Arbic, B.K. et al. Possible link between Earth’s rotation rate and oxygenation. Nat. Geosci. 14, 564–570 (2021). https://doi.org/10.1038/s41561-021-00784-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-021-00784-3
This article is cited by
-
Mid-Proterozoic day length stalled by tidal resonance
Nature Geoscience (2023)
-
Neue Forschungsansätze der Umwelt- und Klimamikrobiologie
BIOspektrum (2023)
-
Growth of continental crust in intra-oceanic and continental-margin arc systems: Analogs for Archean systems
Science China Earth Sciences (2022)
-
Mikrobielle Matten als Fenster in die Erdgeschichte
BIOspektrum (2021)