Abstract
The fate of carbon subducted to mantle depths remains uncertain, yet strongly influences the distribution of terrestrial carbon on geologic timescales. Carbon fluxes into subduction zones are exceptionally high where downgoing plates contain thick sedimentary fans. This study uses volcano geochemistry to assess sedimentary carbon recycling in the high-flux Makran subduction zone, where the Arabian plate subducts northward beneath Eurasia. On the basis of strontium isotope geochemistry and 40Ar–39Ar geochronology, I show that a portion of the submarine Indus Fan entered the Makran Trench, melted and ascended as magmas that erupted in southern Afghanistan. The resulting volcano, composed primarily of carbonate minerals, formed at approximately 3.8 million years ago. The 87Sr/86Sr ratios of the lavas indicate that their magmatic precursors were derived from marine sediments deposited at 28.9 ± 1.4 Ma. This implies that sedimentary carbon was subducted to and returned from mantle depths in less than 27 million years, indicating that magmas can efficiently recycle sedimentary carbon from subducting slabs to the overlying plate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The Ar–Ar geochronology and strontium isotope results are publicly available via EarthChem (https://doi.org/10.26022/IEDA/111960).
References
Plank, T. & Manning, C. E. Subducting carbon. Nature 574, 343–352 (2019).
Behn, M. D., Kelemen, P. B., Hirth, G., Hacker, B. R. & Massonne, H.-J. Diapirs as the source of the sediment signature in arc lavas. Nat. Geosci. 4, 641–646 (2011).
Thomsen, T. B. & Schmidt, M. W. Melting of carbonated pelites at 2.5–5.0 GPa, silicate–carbonatite liquid immiscibility, and potassium–carbon metasomatism of the mantle. Earth Planet. Sci. Lett. 267, 17–31 (2008).
Mallik, A. & Dasgupta, R. Reactive infiltration of MORB-eclogite-derived carbonated silicate melt into fertile peridotite at 3 GPa and genesis of alkalic magmas. J. Petrol. 54, 2267–2300 (2013).
Hirschmann, M. M. Comparative deep Earth volatile cycles: the case for C recycling from exosphere/mantle fractionation of major (H2O, C, N) volatiles and from H2O/Ce, CO2/Ba, and CO2/Nb exosphere ratios. Earth Planet. Sci. Lett. 502, 262–273 (2018).
Kelemen, P. B. & Manning, C. E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl Acad. Sci. USA 112, E3997–E4006 (2015).
Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 75, 183–229 (2013).
Aiuppa, A., Fischer, T. P., Plank, T. & Bani, P. CO2 flux emissions from the Earth’s most actively degassing volcanoes, 2005–2015. Sci. Rep. 9, 5442 (2019).
Clift, P. D. A revised budget for Cenozoic sedimentary carbon subduction. Rev. Geophys. 55, 97–125 (2017).
Burg, J.-P. Geology of the onshore Makran accretionary wedge: synthesis and tectonic interpretation. Earth Sci. Rev. 185, 1210–1231 (2018).
Pang, K.-N., Chung, S.-L., Zarrinkoub, M. H., Chiu, H.-Y. & Li, X.-H. On the magmatic record of the Makran arc, southeastern Iran: insights from zircon U-Pb geochronology and bulk-rock geochemistry. Geochem. Geophys. Geosyst. 15, 2151–2169 (2014).
Hammouda, T. & Keshav, S. Melting in the mantle in the presence of carbon: review of experiments and discussion on the origin of carbonatites. Chem. Geol. 418, 171–188 (2015).
Bell, K. & Simonetti, A. Source of parental melts to carbonatites–critical isotopic constraints. Mineral. Petrol. 98, 77–89 (2010).
Hoernle, K., Tilton, G., Le Bas, M. J., Duggen, S. & Garbe-Schönberg, D. Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate. Contrib. Mineral. Petrol. 142, 520–542 (2002).
Hulett, S. R., Simonetti, A., Rasbury, E. T. & Hemming, N. G. Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nat. Geosci. 9, 904–908 (2016).
Amsellem, E. et al. Calcium isotopic evidence for the mantle sources of carbonatites. Sci. Adv. 6, eaba3269 (2020).
Penney, C. et al. Megathrust and accretionary wedge properties and behaviour in the Makran subduction zone. Geophys. J. Int. 209, 1800–1830 (2017).
Smith, G., McNeill, L., Henstock, T. J. & Bull, J. The structure and fault activity of the Makran accretionary prism. J. Geophys. Res. Solid Earth 117, B07407 (2012).
Pang, K.-N., Teng, F.-Z., Sun, Y., Chung, S.-L. & Zarrinkoub, M. H. Magnesium isotopic systematics of the Makran arc magmas, Iran: implications for crust-mantle Mg isotopic balance. Geochim. Cosmochim. Acta 278, 110–121 (2020).
Motaghi, K., Shabanian, E. & Nozad-Khalil, T. Deep structure of the western coast of the Makran subduction zone, SE Iran. Tectonophysics 776, 228314 (2020).
Simmons, N. A., Myers, S. C. & Johannesson, G. Global-scale P wave tomography optimized for prediction of teleseismic and regional travel times for Middle East events: 2. Tomographic inversion. J. Geophys. Res. Solid Earth 116, B04305 (2011).
Al-Lazki, A. I., Al-Damegh, K. S., El-Hadidy, S. Y., Ghods, A. & Tatar, M. Pn-velocity structure beneath Arabia–Eurasia Zagros collision and Makran subduction zones. Geol. Soc. Spec. Publ. 392, 45–60 (2014).
Tucker, R. D., Belkin, H. E., Schulz, K. J., Peters, S. G. & Buttleman, K. P. Rare Earth Element Mineralogy, Geochemistry, and Preliminary Resource Assessment of the Khanneshin Carbonatite Complex, Helmand Province, Afghanistan (US Geological Survey, 2011).
Tucker, R. D. et al. A major light rare-earth element (LREE) resource in the Khanneshin carbonatite complex, southern Afghanistan. Econ. Geol. 107, 197–208 (2012).
Woolley, A. R. & Church, A. A. Extrusive carbonatites: a brief review. Lithos 85, 1–14 (2005).
Ayuso, R. et al. Preliminary radiogenic isotope study on the origin of the Khanneshin carbonatite complex, Helmand Province, Afghanistan. J. Geochem. Explor. 133, 6–14 (2013).
Horton, F., Nielsen, S., Shu, Y., Gagnon, A. & Blusztajn, J. Thallium isotopes reveal brine activity during carbonatite magmatism. Geochem. Geophys. Geosyst. 22, e2020GC009472 (2021).
Maggi, A., Jackson, J. A., Priestley, K. & Baker, C. A re-assessment of focal depth distributions in southern Iran, the Tien Shan and northern India: do earthquakes really occur in the continental mantle? Geophys. J. Int. 143, 629–661 (2000).
Saadat, S. & Stern, C. R. Petrochemistry and genesis of olivine basalts from small monogenetic parasitic cones of Bazman stratovolcano, Makran arc, southeastern Iran. Lithos 125, 607–619 (2011).
Smith, G. L., McNeill, L. C., Wang, K., He, J. & Henstock, T. J. Thermal structure and megathrust seismogenic potential of the Makran subduction zone. Geophys. Res. Lett. 40, 1528–1533 (2013).
Robert, A. M., Fernàndez, M., Jiménez-Munt, I. & Vergés, J. Lithospheric structure in Central Eurasia derived from elevation, geoid anomaly and thermal analysis. Geol. Soc. Spec. Publ. 427, 271–293 (2017).
Gonzalez, C. M., Gorczyk, W. & Gerya, T. V. Decarbonation of subducting slabs: insight from petrological–thermomechanical modeling. Gondwana Res. 36, 314–332 (2016).
Jarrard, R. D. & Lyle, M. High-resolution geochemical variations at sites 723, 728, and 731: a comparision of X-ray fluorescence and geochemical logs. Proc. Ocean Drill. Prog. Sci. Results 117, 473–498 (1991).
Tsuno, K. & Dasgupta, R. The effect of carbonates on near-solidus melting of pelite at 3 GPa: Relative efficiency of H2O and CO2 subduction. Earth Planet. Sci. Lett. 319, 185–196 (2012).
White, W. M. & Hofmann, A. W. Sr and Nd isotope geochemistry of oceanic basalts and mantle evolution. Nature 296, 821–825 (1982).
White, W. M. Isotopes, DUPAL, LLSVPs, and anekantavada. Chem. Geol. 419, 10–28 (2015).
Clift, P. D. et al. Nd and Pb isotope variability in the Indus River System: implications for sediment provenance and crustal heterogeneity in the Western Himalaya. Earth Planet. Sci. Lett. 200, 91–106 (2002).
Whitney, J. W. Geology, Water and Wind in the Lower Helmand Basin, Southern Afghanistan (US Geological Survey, 2006).
Artemieva, I. M. Global 1° × 1° thermal model TC1 for the continental lithosphere: implications for lithosphere secular evolution. Tectonophysics 416, 245–277 (2006).
Clift, P. D. et al. Development of the Indus Fan and its significance for the erosional history of the Western Himalaya and Karakoram. Geol. Soc. Am. Bull. 113, 1039–1051 (2001).
McArthur, J. M., Howarth, R. J. & Shields, G. A. in The Geologic Time Scale (eds Gradstein, M. A. et al.) 127–144 (Elsevier, 2012).
Platt, J. P., Leggett, J. K., Young, J., Raza, H. & Alam, S. Large-scale sediment underplating in the Makran accretionary prism, southwest Pakistan. Geology 13, 507–511 (1985).
Sleep, N. H. Stagnant lid convection and carbonate metasomatism of the deep continental lithosphere. Geochem. Geophys. Geosyst. 10, Q11010 (2009).
Wyllie, P. J. & Huang, W.-L. Peridotite, kimberlite, and carbonatite explained in the system CaO-MgO-SiO2-CO2. Geology 3, 621–624 (1975).
Carn, S. A., Fioletov, V. E., McLinden, C. A., Li, C. & Krotkov, N. A. A decade of global volcanic SO2 emissions measured from space. Sci. Rep. 7, 44095 (2017).
Werner, C. et al. in Deep Carbon: Past to Present (eds Orcutt, B. N. et al.) 188–236 (Cambridge Univ. Press, 2019).
Brantley, S. L. & Koepenick, K. W. Measured carbon dioxide emissions from Oldoinyo Lengai and the skewed distribution of passive volcanic fluxes. Geology 23, 933–936 (1995).
Zhang, M. et al. Magma-derived CO2 emissions in the Tengchong volcanic field, SE Tibet: implications for deep carbon cycle at intra-continent subduction zone. J. Asian Earth Sci. 127, 76–90 (2016).
Huang, J. & Zhao, D. High-resolution mantle tomography of China and surrounding regions. J. Geophys. Res. Solid Earth 111, B09305 (2006).
House, B. M., Bebout, G. E. & Hilton, D. R. Carbon cycling at the Sunda margin, Indonesia: a regional study with global implications. Geology 47, 483–486 (2019).
Jackson, M. G. & Hart, S. R. Strontium isotopes in melt inclusions from Samoan basalts: implications for heterogeneity in the Samoan plume. Earth Planet. Sci. Lett. 245, 260–277 (2006).
Kuiper, K. F. et al. Synchronizing rock clocks of Earth history. Science 320, 500–504 (2008).
Cahoon, E. B., Streck, M. J., Koppers, A. A. & Miggins, D. P. Reshuffling the Columbia River Basalt chronology—Picture Gorge Basalt, the earliest- and longest-erupting formation. Geology 48, 348–352 (2020).
Koppers, A. A. ArArCALC—software for 40Ar/39Ar age calculations. Comput. Geosci. 28, 605–619 (2002).
Steiger, R. H. & Jäger, E. Subcommission on geochronology: convention on the use of decay constants in geo- and cosmochronology. Earth Planet. Sci. Lett. 36, 359–362 (1977).
Min, K., Mundil, R., Renne, P. R. & Ludwig, K. R. A test for systematic errors in 40Ar/39Ar geochronology through comparison with U/Pb analysis of a 1.1-Ga rhyolite. Geochim. Cosmochim. Acta 64, 73–98 (2000).
Alkhazov, V. Y., Atakishiyev, Z. M. & Azimi, N. A. Geology and mineral resources of the early Quaternary Khanneshin carbonatite volcano (Southern Afghanistan). Int. Geol. Rev. 20, 281–285 (1978).
Plank, T. & Langmuir, C. H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 145, 325–394 (1998).
McDonough, W. F. & Sun, S. S. The composition of the Earth. Chem. Geol. 120, 223–253 (1995).
Acknowledgements
R. Seal at the US Geological Survey granted access to the samples, which were collected by the US Geological Survey and the US Department of Defense Task Force for Business and Stability Operations personnel led by R. Tucker and E. King. The Afghanistan Geological Survey provided access to Soviet reports about the Khanneshin volcano that were essential for the fieldwork. The Afghanistan Ministry of Mines and Petroleum granted permission for sample collection. Combined Forces Special Operations Command - Afghanistan provided logistics and security support. At the time of sample collection, F.H. was an employee of TFBSO. J. Blusztajn and D. Miggins carried out the strontium isotope analyses and Ar–Ar geochronology, respectively. Discussions with S. Nielsen and G. Gaetani improved this paper. This project was supported by the Woods Hole Oceanographic Institution Independent Research & Development Program and a National Science Foundation grant (EAR number 1911699) awarded to F.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information Nature Geoscience thanks Elsa Amsellem, Kwan-Nang Pang and Sabin Zahirovic for their contribution to the peer review of this work. Primary Handling Editor: Rebecca Neely.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
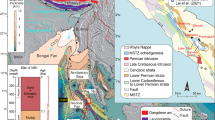
Extended Data Fig. 1 Geologic map of the Khanneshin volcano showing sample locations.
The core of the central vent consists mostly of sövite with abundant fenite xenoliths. Outer portions of the central vent are composed mostly of ankerite-barite carbonatite, inferred to be younger than the sövite because they host sövite xenoliths. Samples (white circles) were collected from two drainages that exit the massif to the northeast. This figure is a modified version of the geologic map published by ref. 27, which is based on ref. 57 and ref. 23.
Extended Data Fig. 2 Mineralogy and petrology of sandstone sample FH-10KH-10.
a, Sawed surface image. The sedimentary clasts are dominantly K-feldspar. Bedding is defined by alternating sandstone and siltstone layers, of which the latter appear darker because they have more interstitial Fe-oxide. The rock is brecciated and crosscut by calcium carbonate veins. B, Closer inspection of a carbonate vein reveals anastomosing networks of smaller veins extending into the sandstone. Beneath the large vein is a porous metasomatized region where cavities are partially filled by carbonate minerals, including minor amounts of REE-carbonates. C, Thin section photomicrograph. Along calcium carbonate vein margins are secondary Fe-Mn-oxides (black).
Extended Data Fig. 3 Mineralogy and petrology of representative sövite samples.
a, KHAN-1, hand sample image. Medium- to coarse-grained calcite carbonatite contains fine-grained biotite. Three small fenite xenoliths are visible in the upper left. b, KHAN-1, PPL. Small fenite xenoliths, like the one imaged here, are composed of biotite. They share sharp and diffuse boundaries with the surrounding calcite matrix. c, KHAN-2, sawed surface image. Larger fenite xenoliths are highly brecciated and crosscut by calcite veins. d, KHAN-2, XPL. Large twinned calcite crystal with Fe-oxide and biotite inclusions. Contact twinning separates the upper and lower portions of the crystal, both of which have subtle lamellae twins. e, KHAN-2, sawed surface image. Large fenite xenoliths have brecciated textures and are crosscut by multiple generations of veins. The white veins are calcite and the green vein, bound by dashed lines, is mostly apatite. f, KHAN-2, XPL. Apatite also forms clusters with biotite in the calcite matrix of sövite samples. g, KHAN-2, XPL. Large brecciated fenite xenoliths have biotite-rich zones adjacent to calcite veins and fine-grained interiors composed of biotite and K-feldspar. h, RT-10K-09, hand sample image. This sövite contains large (>1 cm) phlogopite books intergrown with coarse calcium carbonate. i, RT-10K-09, XPL. Calcite twin lamellae are visible in this sample. Mineral abbreviations: ap = apatite, bio = biotite, cc = calcium carbonate, kfs = K-feldspar. Hand sample and sawed surface images were taken on a stereomicroscope. Thin section images were taken on a polarizing microscope under plain polarized light (PPL) or cross polarized light (XPL).
Extended Data Fig. 4 Mineralogy and petrology of representative ankerite-barite carbonatite samples.
a, FH-10K-08, sawed surface image. Lath-shaped intergrowths of barium, calcium carbonate, and ankerite form the matrix of this carbonatite. Fe-Mn oxides are present and REE-carbonates line the walls of cavities. b, FH-10K-08, PPL. The complex textures of the barium-calcium carbonate intergrowths—perhaps pseudomorphs after witherite—can be observed. Tetraferriphlogopite also exists in this sample as a minor phase. c, RT-10K-03, sawed surface image. Ankerite-barite carbonatites also contain fenite xenoliths, as shown here. REE carbonates appear yellow. d, RT-10K-03, PPL. Ankerite-barite carbonatites exhibit varied textures. Here, barite (outlined with a dashed line) and ankerite are surrounded by a finer-grained matrix of calcium carbonate. e, RT-10K-11, sawed surface image. Intergrowths of ankerite and Fe-oxides often form clusters. f, RT-10K-11, PPL. Tabular barite crystals occur in intimate association with ankerite and Fe-oxide. g, RT-10K-07, sawed surface image. Some samples are relatively homogeneous on the cm scale. Here, ankerite (grey) surrounds intergrowths of barite (white) and calcium carbonate (also white). Yellow regions contain REE minerals hosted in cavities. h, RT-10K-07, PPL. Tetraferriphlogopite is sometime rimmed by REE-carbonate minerals and tends to be associated with barite-calcium carbonate intergrowths. i, RT-10K-03, XPL. Solitary fluorite crystals (isotropic) exist in some ankerite-barite carbonatites and are rimmed by calcium carbonate. Mineral abbreviations: ank = ankerite, ba = barite cc = calcium carbonate, fl = fluorite, tfp = tetraferriphlogopite, REE = rare earth element carbonate minerals.
Extended Data Fig. 5 Mineralogy and petrology of REE-rich ankerite barite carbonatites.
a, RT-11K-4A0, sawed surface image. Aggregates of yellow REE-carbonates in a matrix of ankerite, barite, and calcite. Clusters of REE-carbonate and strontianite form lighter yellow clusters. b, RT- 11K-4A0, XPL. Contact between REE-carbonate aggregates and ankerite-barite-calcite matrix. c, RT-11K-5B3B, sawed surface image. Ankerite (dark brown patches) plus calcium carbonate (grey) zones alternate with barite, strontianite, and REE-carbonate aggregates. d, RT-11K-5B3B, XPL. Spherulitic acicular strontianite occurs in association with ankerite, calcium carbonate, and barite. e, RT-11K-2B, XPL. In some cases, subhedral domains of barite and ankerite are rimmed by zones of ankerite, strontianite plus REE-carbonates, and apatite. f, RT-11K-5B6C, XPL. Mn oxides occur as veins and clusters. Here, tabular Mn-oxide in a vein contains interstitial calcium carbonate, which grades into intergrowths of strontianite and REE-carbonate minerals. Mineral abbreviations: ank = ankerite, ba = barite, cc = calcium carbonate, REE = rare earth element carbonate minerals, str = strontianite.
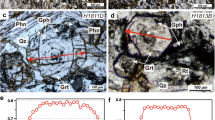
Extended Data Fig. 6 40Ar/39Ar plateau diagrams.
a–c, Step heating analyses of RT-10K-09 phlogopite aliquots 1–3, respectively.
Extended Data Fig. 7 Trace element model.
a, The trace element concentrations in the Khanneshin carbonatite samples vary by roughly 1–2 orders of magnitude for each element (grey shaded region). In general, sövite samples are less enriched than ankerite-barite carbonatites. Colored lines represent mean compositions for Khanneshin sövites and ankerite-barite carbonatites with (“REE”) and without (“A-B C”) abundant REE minerals. b, The modeled composition of carbonatitic melt derived from subducted Makran sedimentary material—assumed to equal average Indus Fan sediments58—and Khanneshin carbonatite samples (grey shaded region) have similar trace element patterns. The model assumes that 30% melting of subducted sediments produced a carbonated silicate magma that separated into immiscible carbonatitic and silicate magmas during ascent. See Supplementary Information text for details. All values are normalized to primitive mantle59.
Supplementary information
Supplementary Information
Sample descriptions and trace element model.
Supplementary Data 1
The complete 40Ar–39Ar geochronology report received from Oregon State University Argon Geochronology Laboratory for RT-10K-09 phlogopite aliquot 1.
Supplementary Data 2
The complete 40Ar–39Ar geochronology report received from Oregon State University Argon Geochronology Laboratory for RT-10K-09 phlogopite aliquot 2.
Supplementary Data 3
The complete 40Ar–39Ar geochronology report received from Oregon State University Argon Geochronology Laboratory for RT-10K-09 phlogopite aliquot 3.
Rights and permissions
About this article
Cite this article
Horton, F. Rapid recycling of subducted sedimentary carbon revealed by Afghanistan carbonatite volcano. Nat. Geosci. 14, 508–512 (2021). https://doi.org/10.1038/s41561-021-00764-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-021-00764-7
This article is cited by
-
Recycled carbonates elevate the electrical conductivity of deeply subducting eclogite in the Earth’s interior
Communications Earth & Environment (2023)
-
Resurfacing subducted carbon
Nature Reviews Earth & Environment (2021)
-
Hadal aragonite records venting of stagnant paleoseawater in the hydrated forearc mantle
Communications Earth & Environment (2021)