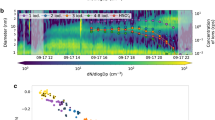
Abstract
New particle formation is globally one of the major sources of aerosol particles and cloud condensation nuclei. As primary emissions are a minor contributor to particle concentrations, secondary new particle formation processes are probably key in determining Antarctic aerosol number concentrations. However, our knowledge of new particle formation and its mechanisms in Antarctica is very limited. Here we study summertime open ocean and coastal new particle formation in the Antarctic Peninsula region based on both ship and station measurements. The rates of particle formation relative to sulfuric acid concentrations, as well as the sulfuric acid dimer-to-monomer ratios, were similar to those seen for sulfuric acid–dimethylamine–water nucleation. Numerous sulfuric acid–amine peaks were identified during new particle formation events, providing evidence that alkylamines were the bases that facilitated sulfuric acid nucleation. Most new particle formation events occurred in air masses arriving from the ice-covered Weddell Sea and its marginal ice zone, which are an important source of volatile sulfur and alkylamines. This nucleation mechanism is more efficient than the ion-induced sulfuric acid–ammonia pathway previously observed in Antarctica, and one that can occur rapidly under neutral conditions. This hitherto overlooked pathway to biologically driven aerosol formation should be considered for estimating aerosol and cloud condensation nuclei numbers in ocean–sea ice–aerosols–climate feedback models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data supporting this publication are openly available from the UBIRA eData repository at https://doi.org/10.25500/edata.bham.00000400. Daily sea-ice concentrations49 are available from the NSIDC at https://doi.org/10.7265/N5K072F8.
Code availability
Code required to produce the figures is available from J.B. (J.brean@bham.ac.uk) upon reasonable request.
References
Turner, J. et al. Antarctic climate change during the last 50 years. Int. J. Climatol. 25, 279–294 (2005).
Turner, J. et al. Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535, 411–415 (2016).
Siegert, M. et al. The Antarctic Peninsula under a 1.5 °C global warming scenario. Front. Environ. Sci. 7, 107 (2019).
Turton, J. V., Kirchgaessner, A., Ross, A. N. & King, J. C. The spatial distribution and temporal variability of föhn winds over the Larsen C ice shelf, Antarctica. Q. J. R. Meteorol. Soc. 144, 1169–1178 (2018).
IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2014).
Carslaw, K. S. et al. Large contribution of natural aerosols to uncertainty in indirect forcing. Nature 503, 67–71 (2013).
Weller, R., Schmidt, K., Teinilä, K. & Hillamo, R. Natural new particle formation at the coastal Antarctic site Neumayer. Atmos. Chem. Phys. 15, 11399–11410 (2015).
Jang, E. et al. New particle formation events observed at the King Sejong Station, Antarctic Peninsula - Part 2: link with the oceanic biological activities. Atmos. Chem. Phys. 19, 7595–7608 (2019).
O’Dowd, C. D. et al. Biogenic sulphur emissions and inferred non-sea-salt-sulphate cloud condensation nuclei in and around Antarctica. J. Geophys. Res. Atmos. 102, 12839–12854 (1997).
Dall’Osto, M. et al. Antarctic sea ice region as a source of biogenic organic nitrogen in aerosols. Sci. Rep. 7, 6047 (2017).
Dall’Osto, M. et al. Simultaneous detection of alkylamines in the surface ocean and atmosphere of the antarctic sympagic environment. ACS Earth Space Chem. 3, 854–862 (2019).
Lachlan-Cope, T. et al. On the annual variability of Antarctic aerosol size distributions at Halley research station. Atmos. Chem. Phys. 20, 4461–4476 (2020).
Hoffmann, E. H. et al. An advanced modeling study on the impacts and atmospheric implications of multiphase dimethyl sulfide chemistry. Proc. Natl Acad. Sci. USA 113, 11776–11781 (2016).
Lee, S. H. et al. New particle formation in the atmosphere: from molecular clusters to global climate. J. Geophys. Res. Atmos. https://doi.org/10.1029/2018JD029356 (2019).
Chen, H. & Finlayson-Pitts, B. J. New particle formation from methanesulfonic acid and amines/ammonia as a function of temperature. Environ. Sci. Technol. 51, 243–252 (2017).
Bork, N., Elm, J., Olenius, T. & Vehkamäki, H. Methane sulfonic acid-enhanced formation of molecular clusters of sulfuric acid and dimethyl amine. Atmos. Chem. Phys. 14, 12023–12030 (2014).
Rose, C. et al. Observations of biogenic ion-induced cluster formation in the atmosphere. Sci. Adv. 4, eaar5218 (2018).
Sipilä, M. et al. Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3. Nature 537, 532–534 (2016).
Jokinen, T. et al. Ion-induced sulfuric acid–ammonia nucleation drives particle formation in coastal Antarctica. Sci. Adv. 4, eaat9744 (2018).
Yao, L. et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science 361, 278–281 (2018).
Kirkby, J. et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476, 429–435 (2011).
Almeida, J. et al. Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere. Nature 502, 359–363 (2013).
Olenius, T. et al. New particle formation from sulfuric acid and amines: comparison of monomethylamine, dimethylamine, and trimethylamine. J. Geophys. Res. 122, 7103–7118 (2017).
Kirkby, J. et al. Ion-induced nucleation of pure biogenic particles. Nature 533, 521–526 (2016).
Kyrö, E.-M. et al. Antarctic new particle formation from continental biogenic precursors. Atmos. Chem. Phys. 13, 3527–3546 (2013).
Järvinen, E. et al. Seasonal cycle and modal structure of particle number size distribution at Dome C, Antarctica. Atmos. Chem. Phys. 13, 7473–7487 (2013).
Dal Maso, M. et al. Formation and growth of fresh atmospheric aerosols: eight years of aerosol size distribution data from SMEAR II, Hyytiälä, Finland. Boreal Environ. Res. 10, 323–336 (2005).
Kupiainen, O., Ortega, I. K., Kurté, T. & Vehkamäki, H. Amine substitution into sulfuric acid-ammonia clusters. Atmos. Chem. Phys. 12, 3591–3599 (2012).
Kürten, A. et al. Neutral molecular cluster formation of sulfuric acid–dimethylamine observed in real time under atmospheric conditions. Proc. Natl Acad. Sci. USA 111, 15019–15024 (2014).
Ge, X., Wexler, A. S. & Clegg, S. L. Atmospheric amines–part II. Thermodynamic properties and gas/particle partitioning. Atmos. Environ. 45, 561–577 (2011).
Kim, J. et al. New particle formation events observed at King Sejong Station, Antarctic Peninsula–part 1: physical characteristics and contribution to cloud condensation nuclei. Atmos. Chem. Phys. 19, 7583–7594 (2019).
Hodshire, A. L. et al. The potential role of methanesulfonic acid (MSA) in aerosol formation and growth and the associated radiative forcings. Atmos. Chem. Phys. 19, 3137–3160 (2019).
Bianchi, F. et al. Highly oxygenated organic molecules (HOM) from gas-phase autoxidation involving peroxy radicals: a key contributor to atmospheric aerosol. Chem. Rev. 119, 3472–3509 (2019).
Mcfiggans, G. et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 565, 587–593 (2019).
Paasonen, P. et al. On the formation of sulphuric acid–amine clusters in varying atmospheric conditions and its influence on atmospheric new particle formation. Atmos. Chem. Phys. 12, 9113–9133 (2012).
Glasoe, W. A. et al. Sulfuric acid nucleation: an experimental study of the effect of seven bases. J. Geophys. Res. Atmos. 175, 1933–1950 (2015).
Ortega, I. K. et al. From quantum chemical formation free energies to evaporation rates. Atmos. Chem. Phys. 12, 225–235 (2012).
Jameson, E. et al. Metagenomic data-mining reveals contrasting microbial populations responsible for trimethylamine formation in human gut and marine ecosystems. Microb. Genom. 2, https://doi.org/10.1099/mgen.0.000080 (2016).
Oren, A. Formation and breakdown of glycine betaine and trimethylamine in hypersaline environments. Ant. Van Leeuw. 58, 291–298 (1990).
Burg, M. B., Ferraris, J. D., Burg, M. B. & Ferraris, J. D. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 283, 7309–7313 (2008).
Jung, J. et al. Characteristics of methanesulfonic acid, non-sea-salt sulfate and organic carbon aerosols over the Amundsen Sea, Antarctica. Atmos. Chem. Phys. 20, 5405–5424 (2020).
Simon, M. et al. Detection of dimethylamine in the low pptv range using nitrate chemical ionization atmospheric pressure interface time-of-flight (CI-APi-TOF) mass spectrometry. Atmos. Meas. Technol. 9, 2135–2145 (2016).
Brean, J. et al. Observations of highly oxidised molecules and particle nucleation in the atmosphere of Beijing. Atmos. Chem. Phys. 19, 14933–14947 (2019).
Kulmala, M. et al. Measurement of the nucleation of atmospheric aerosol particles. Nat. Protoc. 7, 1651–1667 (2012).
Korhonen, H., Kerminen, V. M., Kokkola, H. & Lehtinen, K. E. J. Estimating atmospheric nucleation rates from size distribution measurements: analytical equations for the case of size dependent growth rates. J. Aerosol Sci. 69, 13–20 (2014).
Nieminen, T., Lehtinen, K. E. J. & Kulmala, M. Sub-10 nm particle growth by vapor condensation-effects of vapor molecule size and particle thermal speed. Atmos. Chem. Phys. 10, 9773–9779 (2010).
Kurtén, T., Noppel, M., Vehkamäki, H., Salonen, M. & Kulmala, M. Quantum chemical studies of hydrate formation of H2SO4 and HSO4−. Boreal Environ. Res. 12, 431–453 (2007).
Yli-Juuti, T. et al. Growth rates of nucleation mode particles in Hyytiälä during 2003-2009: variation with particle size, season, data analysis method and ambient conditions. Atmos. Chem. Phys. 11, 12865–12886 (2011).
Ezraty, R., Girard-Ardhuin, F., Piolle, J. F. & Heygster, L. K. G. Arctic and Antarctic Sea-Ice Concentration and Arctic Sea Ice Drift Estimated from Special Sensor Microwave Imager Data Version 2.1 (Département d’Océanographie Physique et Spatiale, IFREMER & Univ. Bremen, 2007).
Massoli, P. et al. Ambient measurements of highly oxidized gas-phase molecules during the Southern Oxidant and Aerosol Study (SOAS) 2013. ACS Earth Space Chem. 2, 653–672 (2018).
Acknowledgements
We thank the Spanish Armada, and particularly the captains and crew of the BIO A-33 Hesperides, for their invaluable collaboration. We are also indebted to the UTM, and especially M. Ojeda, for logistic and technical support on the Antarctic Spanish BAE JC1. We also thank A. Sotomayor for help with mapping. This study was funded by the Spanish Ministry of Economy (grant number PI‐ICE‐CTM 2017–89117‐R and RYC-2012-11922, both awarded to M.D.’O.). This work was also supported by the National Centre for Atmospheric Science funded by the UK Natural Environment Research Council (grant number R8/H12/83/011 to R.M.H. and D.C.S.B., which also supported a studentship (ncasstu009) for J.B.).
Author information
Authors and Affiliations
Contributions
M.D.’O., J.B. and D.C.S.B. made the field measurements. M.D.’O. organized the campaign and the cruise. J.B. processed the data and led the data interpretation and produced the first draft of the paper. R.M.H., Z.S. and R.S. supported the data interpretation and manuscript drafting.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Geoscience thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis; Xujia Jiang.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characteristics of amine signals.
(a–d) correlations of each ammonia/amine measurement as the form (Am)(HNO3)NO3- and (Am)(HNO3)2NO3-, where Am is NH3, methylamine, C2 or C4 amine, coloured by date for (a) C2 amines, (b) C4 amines, (c) methylamine and (d) ammonia, and (e-f) mass spectral peak fits for C2 amine clustered with the nitrate dimer and trimer.
Extended Data Fig. 2 GR4.5-10 by different methods.
Bars corresponding to ‘Calc H2SO4 + MSA + HIO3’ and ‘Calc H2SO4’ represent the theoretical growth rate as calculated by ref. 46 due to several vapours, and H2SO4 respectively, the rightmost bars show growth rates calculated by the lognormal fitting method applied to NanoSMPS data. Errors on growth rates fitted to SMPS data are ±50%, errors on calculated growth rates are +100%/-50%.
Extended Data Fig. 3 Diurnal cycles of ions for station measurements.
Separated by NPF and non-NPF days, for (a) oxygenated organics, containing C5 and C6 oxygenated organics, (b) dicarboxylic acids, (c) iodic acid, (d) methanesulphonic acid, and (e) SO3- and SO5- ions. Shaded regions show 95% confidence regions on the mean.
Extended Data Fig. 4 NO3- CI-APi-ToF PMF results for 8 factors.
showing (a) diurnals, where shaded region shows 1 standard deviation on the mean, (b) time series, and (c) mass spectra per factor. Data included 300 peaks between 150 – 400 m/Q for 1 week of CI-APi-ToF data. Q/Qexp for this solution = 1.004.
Extended Data Fig. 5 Measurement locations.
showing the measurement site (a) within Antarctica, and (b) within the Antarctic peninsula. BAE JC1 is the location for station measurements, and the coloured line shows the ship track, where red signifies that an NPF event was occurring.
Extended Data Fig. 6 Potential new particle sources during cruise measurements.
Signals of gas phase H2SO4, MSA, HIO3, oxygenated organic molecules, C2 amines, and C4 amines. ‘NPF’ refers to periods where NPF was actively occurring (presuming a 0.5 nm h-1 growth rate below 4.5 nm), ‘Non NPF’ refers to the rest of the measurement period. Error bars represent 1 standard error on measured values.
Extended Data Fig. 7 Features of sulphuric acid clusters.
Showing (a) time series, (b) diurnal cycles and (c) relative signals of a series of sulphuric acid clusters. Signals for the time series and diurnals have been normalised to a maximum of 1. Purple line shows the sulphuric acid dimer for comparison, and the black line shows the average of all these dimer peaks. Nucleation events occurred on 28/02 and 05/03.
Extended Data Fig. 8 HR fits for each cluster.
Titles show the assigned cluster formula assigned to the red line. The purple and orange dashed line show unassigned peaks. The sum of these cluster and unknown peak fits is shown with the blue line, and the raw mass spectral data with the green points. Clusters seen with multiple nitrogen atoms are presumed to contain multiple bases.
Rights and permissions
About this article
Cite this article
Brean, J., Dall’Osto, M., Simó, R. et al. Open ocean and coastal new particle formation from sulfuric acid and amines around the Antarctic Peninsula. Nat. Geosci. 14, 383–388 (2021). https://doi.org/10.1038/s41561-021-00751-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-021-00751-y
This article is cited by
-
Significant contributions of trimethylamine to sulfuric acid nucleation in polluted environments
npj Climate and Atmospheric Science (2023)
-
Atmospheric new particle formation from the CERN CLOUD experiment
Nature Geoscience (2023)
-
The critical role of dimethylamine in the rapid formation of iodic acid particles in marine areas
npj Climate and Atmospheric Science (2022)
-
Spectroscopic characterization of two peroxyl radicals during the O2-oxidation of the methylthio radical
Communications Chemistry (2022)
-
Oceanic phytoplankton are a potentially important source of benzenoids to the remote marine atmosphere
Communications Earth & Environment (2021)