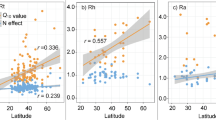
Abstract
Quantifying soil carbon dynamics is of utmost relevance in the context of global change because soils play an important role in land–atmosphere gas exchange. Our current understanding of both present and future carbon dynamics is limited because we fail to accurately represent soil processes across temporal and spatial scales, partly because of the paucity of data on the relative importance and hierarchical relationships between microbial, geochemical and climatic controls. Here, using observations from a 3,000-kyr-old soil chronosequence preserved in alluvial terrace deposits of the Merced River, California, we show how soil carbon dynamics are driven by the relationship between short-term biotic responses and long-term mineral weathering. We link temperature sensitivity of heterotrophic respiration to biogeochemical soil properties through their relationship with microbial activity and community composition. We found that soil mineralogy, and in particular changes in mineral reactivity and resulting nutrient availability, impacts the response of heterotrophic soil respiration to warming by altering carbon inputs, carbon stabilization, microbial community composition and extracellular enzyme activity. We demonstrate that biogeochemical alteration of the soil matrix (and not short-term warming) controls the composition of microbial communities and strategies to metabolize nutrients. More specifically, weathering first increases and then reduces nutrient availability and retention, as well as the potential of soils to stabilize carbon.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedlingstein, P. et al. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 27, 511–526 (2014).
Todd-Brown, K. E. O. et al. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 10, 1717–1736 (2013).
Doetterl, S. et al. Soil carbon storage controlled by interactions between geochemistry and climate. Nat. Geosci. 8, 780–783 (2015).
Hartmann, J., Moosdrof, N., Lauerwald, R., Hinderer, M. & West, A. J. Global chemical weathering and associated P-release—the role of lithology, temperature and soil properties. Chem. Geol. 363, 145–163 (2014).
Lawrence, C. R., Harden, J. W., Xu, X., Schulz, M. S. & Trumbore, S. E. Long-term controls on soil organic carbon with depth and time: a case study from the Cowlitz River Chronosequence, WA USA. Geoderma 247–248, 73–87 (2015).
Hicks Pries, C. E., Castanha, C., Porras, R. & Torn, M. S. The whole-soil carbon flux in response to warming. Science 355, 1420–1423 (2017).
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006).
Ciais, P. et al. in Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) 465–570 (Cambridge Univ. Press, 2013).
Friend, A. D. et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl Acad. Sci. USA 111, 3280–3285 (2014).
Oertel, C., Matschullat, J., Zurba, K., Zimmermann, F. & Erasmi, S. Greenhouse gas emissions from soils—a review. Geochemistry 76, 327–352 (2016).
Leblans, N. I. W. et al. Phenological responses of Icelandic subarctic grasslands to short-term and long-term natural soil warming. Global Change Biol. 23, 4932–4945 (2017).
Knorr, W., Prentice, I. C., House, J. I. & Holland, E. A. Long-term sensitivity of soil carbon turnover to warming. Nature 433, 298–301 (2005).
Frey, S. D., Lee, J., Melillo, J. M. & Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change 3, 395–398 (2013).
Cotrufo, M. F. et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 8, 776–779 (2015).
Opolot, E. & Finke, P. A. Evaluating sensitivity of silicate mineral dissolution rates to physical weathering using a soil evolution model (SoilGen2.25). Biogeosciences 12, 6791–6808 (2015).
Barré, P., Fernandez-Ugalde, O., Virto, I., Velde, B. & Chenu, C. Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: incomplete knowledge and exciting prospects. Geoderma 235–236, 382–395 (2014).
Vitousek, P. M. & Chadwick, O. A. Pedogenic thresholds and soil process domains in basalt-derived soils. Ecosystems 16, 1379–1395 (2013).
Six, J., Conant, R. T., Paul, E. & Paustian, K. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241, 155–176 (2002).
Moore, J., Macalady, J. L., Schulz, M. S., White, A. F. & Brantley, S. L. Shifting microbial community structure across a marine terrace grassland chronosequence, Santa Cruz, California. Soil Biol. Biochem 42, 21–31 (2010).
Griepentrog, M. et al. Nitrogen deposition promotes the production of new fungal residues but retards the decomposition of old residues in forest soil fractions. Global Change Biol. 20, 327–340 (2014).
Bodé, S., Fancy, R. & Boeckx, P. Stable isotope probing of amino sugars—a promising tool to assess microbial interactions in soils. Rapid Commun. Mass Spectrom. 27, 1367–1379 (2013).
Bai, Z., Bodé, S., Huygens, D., Zhang, X. & Boeckx, P. Kinetics of amino sugar formation from organic residues of different quality. Soil Biol. Biochem. 57, 814–821 (2013).
Engelking, B., Flessa, H. & Joergensen, R. G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 39, 2111–2118 (2007).
Fabian, J., Zlatanovic, S., Mutz, M. & Premke, K. Fungal–bacterial dynamics and their contribution to terrigenous carbon turnover in relation to organic matter quality. ISME J. 11, 415–425 (2017).
Monard, C., Gantner, S., Bertilsson, S., Hallin, S. & Stenlid, J. Habitat generalists and specialists in microbial communities across a terrestrial–freshwater gradient. Sci. Rep. 6, 37719 (2016).
de Graaff, M. A., Classen, A. T., Castro, H. F. & Schadt, C. W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 188, 1055–1064 (2010).
de Vries, F. T. et al. Extensive management promotes plant and microbial nitrogen retention in temperate grassland. PLoS ONE 7, e51201 (2012).
Wardle, D. A. et al. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 (2004).
Sinsabaugh, R. L. & Shah, J. J. F. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102, 31–43 (2011).
Barré, P. et al. The energetic and chemical signatures of persistent soil organic matter. Biogeochemistry 130, 1–12 (2016).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105 (2017).
Allison, S. D., Wallenstein, M. D. & Bradford, M. A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 3, 336–340 (2010).
Turner, S. et al. Mineralogical impact on long-term patterns of soil nitrogen and phosphorus enzyme activities. Soil Biol. Biochem. 68, 31–43 (2014).
Sinsabaugh, R. L., Hill, B. H. & Shah, J. J. F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462, 795–598 (2009).
Birkhofer, K. et al. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS ONE 7, e43292 (2012).
Fierer, N., Brafdorf, M. A. & Jackson, R. B. Towards an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Dungait, J. A., Hopkins, D. W., Gregory, A. S. & Whitmore, A. P. Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biol. 18, 1781–1796 (2012).
Sierra, C. A., Müller, M., Metzler, H., Manzoni, S. & Trumbore, S. E. The muddle of ages, turnover, transit, and residence times in the carbon cycle. Global Change Biol. 23, 1763–1773 (2017).
Bradford, M. A. et al. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Change 6, 751–758 (2016).
Sistla, S. A. et al. Long-term warmin restructures Arctic tundra without changing net soil carbon storage. Nature 497, 615–618 (2013).
Doetterl, S. et al. Erosion, deposition and soil C: a review of process-level controls, experimental tools and models to address C cycling in dynamic landscapes. Earth Sci. Rev. 154, 102–122 (2016).
Guidelines for Soil Description 4th edn (FAO, 2006).
IUSS Working Group WRB. World Reference Base for Soil Resources 2014: Update 2015 World Soil Resources Reports 106 (FAO, 2015).
Harden, J. W. Soils Developed in Granitic Alluvium near Merced, California USGS Bulletin 1590–A (US Government Printing Office, 1987).
Harden, J. W. Genetic interpretations of elemental and chemical differences in a soil chronosequence, California. Geoderma 43, 179–193 (1988).
Marchand D. E. & Allwardt, A. Late Cenozoic Stratigraphic Units in Northeastern San Joaquin Valley, California USGS Bulletin 170 (US Government Printing Office, 1981).
White, A. F. in Chemical Weathering Rates of Silicate Minerals Reviews in Mineralogy Vol. 31 (eds White, A. F. & Brantley, S. L.) 407–458 (Mineralogical Society of America, 1995).
White, A. F. et al. Chemical weathering rates of a soil chronosequence on granitic alluvium: III. Hydrochemical evolution and contemporary solute fluxes and rates. Geochim. Cosmochim. Acta 69, 1975–1996 (2005).
Baisden, W. T. et al. A multiisotope C and N modeling analysis of soil organic matter turnover and transport as a function of soil depth in a California annual grassland soil chronosequence. Global Biogeochem. Cycles 16, 1135 (2002).
Smith, G. I., Bischoff, J. L. & Bradbury, J. P. in An 800,000-year Paleoclimatic Record from Core Ol-92: Owens Lake, Southeast California GSA Special Paper Vol. 317 (eds Smith, G. I. & Bischoff, J. L.) 143–160 (Geological Society of America, 1997).
Rheis, M. et al. A half-million year record of palaeoclimate from the Lake Manix Core, Mojave Desert, California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 365, 11–37 (2012).
Paruleo, J. M., Epstein, H. E., Lauenroth, W. K. & Burke, I. C. ANPP estimates from NDVI for the central grassland region of the United States. Ecology 78, 953–958 (1997).
Gorelick, N. et al. Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27 (2018).
Berhe, A. A. Decomposition of organic substrates at eroding vs. depositional landform positions. Plant Soil 350, 261–280 (2012).
Harden, J. W. An index of soil development from field descriptions: examples from a chronosequence in central California. Geoderma 28, 1–28 (1982).
Beuselinck, L. et al. Grain-size analysis by laser diffractometry: comparison with the sieve-pipette method. Catena 32, 193–208 (1998).
Miller, B. A. & Schaetzl, R. J. Precision of soil particle size analysis using laser diffractometry. Soil Sci. Soc. Am. J. 76, 1719–1727 (2011).
Blake, G. R. & Hartge, K. H. in Methods of Soil Analysis, Part I. Physical and Mineralogical Methods Agronomy Monograph Vol. 9 (ed. Klute, A.) 363–375 (American Society of Agronomy—Soil Science Society of America, Madison, 1986).
Herbillon, A. J. Chemical estimation of weatherable minerals present in the diagnostic horizons of low activity clay soils. In: Proc. 8th Int. Soil Classification Workshop Part 1 (eds Beinroth, F. H., Camargo, M. N. & Eswaran, H.) 39–48 (EMBRAPA-SNLCS/SMSS/USDA-SCS/UPR, 1986).
Chao, T. T. & Sanzolone, R. F. Decomposition techniques. J. Geochem. Explor. 44, 65–106 (1992).
Mehra, O. P. & Jackson, M. L. Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. Clay Miner. 5, 317–327 (1960).
Hendershot, W. H, Lalande, H. & Duquette, M. in Soil Sampling and Methods of Analysis (ed. Carter, M. R.) 167–176 (Lewis Publishers, Boca Raton, 1993).
Robert, M. & Tessier, D. Méthode de préparation des argiles des sols pour des études minéralogiques. Ann. Agron. 25, 859–882 (1974).
Brindley, G. W. & Brown, G. Crystal Structures of Clay Minerals and Their Identification Mineralogical Society Monograph 5 (Mineralogical Society, London, 1980).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Stewart, C. E., Paustian, K., Plante, A. F., Conant, R. T. & Six, J. Soil carbon saturation: linking concept and measurable carbon pools. Soil Sci. Soc. Am. J. 72, 379–392 (2008).
Paul, E. A., Morris, S. J. & Boehm, S. in Assessment Methods for Soil Carbon Advances in Soil Science (eds Lal, R. et al.) 193–206 (CRC/Lewis Publishers, Boca Raton, 2001).
Xu, X. et al. Modifying a sealed tube zinc reduction method for preparation of AMS graphite targets: reducing background and attaining high precision. Nucl. Instrum. Meth B 259, 320–329 (2007).
Stuiver, M. & Polach, H. A. Discussion reporting of 14C data. Radiocarbon 19, 355–363 (1977).
Bodé, S., Denef, K. & Boeckx, P. Development and evaluation of a high-performance liquid chromatography/isotope ratio mass spectrometry methodology for 13C analyses of amino sugars in soil. Rapid Commun. Mass Spectrom. 23, 2519–2526 (2009).
Kaiser, C. et al. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 187, 843–858 (2010).
Schindlbacher, A., Schnecker, J., Takriti, M., Borken, W. & Wanek, W. Microbial physologiy and soil CO2 efflux after 9 years of soil warming in a temperate forest—no indications for thermal adaptions. Global Change Biol. 21, 4265–4277 (2015).
Sinsabaugh, R. L. et al. Stichiometry of soil enzyme activtiy at global scale. Ecol. Lett. 11, 1252–1264 (2008).
Lashermes, G., Gainvors-Claisse, A., Recous, S. & Bertrand, I. Enzymatic strategies and carbon use efficiency of a litter-decomposing fungus grown on maize leaves, stems, and roots. Front. Microbiol. 7, 1315 (2016).
IBM Corp. IBM SPP Statistics for Windows. v.24.0 (IBM, 2016).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016); http://www.R-project.org/.
Oksanen, J. et al. vegan: Community Ecology Package. R Package v.2.4-3 (2017).
Acknowledgements
This research was financed in the framework of the BELSPO funded research action P7 as part of project P7/24 ‘SOGLO’. Further financial support has been given by: UC Merced, UC Louvain, ETH Zurich and Augsburg University. Special thanks to H. Maclean (Maclean Scientific Editing) for language proof reading, to M. Schulz (US Geological Survey) for providing a USGS internal review of this manuscript, as well as the Soil and Water Conservation Society and the US Department of Agriculture for imagery of microbial communities used in Supplementary Fig. 2.
Author information
Authors and Affiliations
Contributions
S.D. and A.A.B. designed the research and co-authors are listed in alphabetical order. S.D., A.A.B., J.Six, K.V.O. and J.W.H. conducted sampling campaigns. S.D., C.A., L.F., J.Schnecker, J.Six, C.V., E.N. and M.G. collected, analysed and interpreted the data. All authors contributed to the writing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Data and Figures
Supplementary Dataset
Raw data for XRD spectra on total and clay mineralogy, including an explanatory help text file.
Rights and permissions
About this article
Cite this article
Doetterl, S., Berhe, A.A., Arnold, C. et al. Links among warming, carbon and microbial dynamics mediated by soil mineral weathering. Nature Geosci 11, 589–593 (2018). https://doi.org/10.1038/s41561-018-0168-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-018-0168-7
This article is cited by
-
The interplay between microbial communities and soil properties
Nature Reviews Microbiology (2024)
-
Geomorphic controls on the abundance and persistence of soil organic carbon pools in erosional landscapes
Nature Geoscience (2024)
-
Different contributing processes in bacterial vs. fungal necromass affect soil carbon fractions during plant residue transformation
Plant and Soil (2024)
-
PFOA and PFOS induces mineralization of soil organic carbon by accelerating the consumption of dissolved organic carbon
Carbon Research (2024)
-
Land-use induced soil carbon stabilization at the expense of rock derived nutrients: insights from pristine Andean soils
Scientific Reports (2023)