Abstract
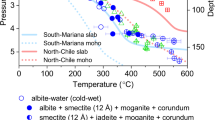
Water is the most abundant volatile component in the Earth. It continuously enters the mantle through subduction zones, where it reduces the melting temperature of rocks to generate magmas. The dehydration process in subduction zones, which determines whether water is released from the slab or transported into the deeper mantle, is an essential component of the deep water cycle. Here we use in situ and time-resolved high-pressure/high-temperature synchrotron X-ray diffraction and infrared spectra to characterize the structural and chemical changes of the clay mineral kaolinite. At conditions corresponding to a depth of about 75 km in a cold subducting slab (2.7 GPa and 200 °C), and in the presence of water, we observe the pressure-induced insertion of water into kaolinite. This super-hydrated phase has a unit cell volume that is about 31% larger, a density that is about 8.4% lower than the original kaolinite and, with 29 wt% H2O, the highest water content of any known aluminosilicate mineral in the Earth. As pressure and temperature approach 19 GPa and about 800 °C, we observe the sequential breakdown of super-hydrated kaolinite. The formation and subsequent breakdown of super-hydrated kaolinite in cold slabs subducted below 200 km leads to the release of water that may affect seismicity and help fuel arc volcanism at the surface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Keken, P. E., Hacker, B. R., Syracuse, E. M. & Abers, G. A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. 116, B10401 (2011)
Wallace, P. J. Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geoth. Res. 140, 217–240 (2005).
Tatsumi, Y. & Eggins, S. Subduction Zone Magmatism (Blackwell Scientific, Oxford, 1995).
Mibe, K., Fujii, T. & Yasuda, A. Control of the location of the volcanic front in island arcs by aqueous fluid connectivity in the mantle wedge. Nature 401, 259–262 (1999).
Hattori, K. H. & Guillot, S. Volcanic fronts form as a consequence of serpentinite dehydration in the forearc mantle wedge. Geology 31, 525–528 (2003).
Ohtani, E. Hydrous minerals and the storage of water in the deep mantle. Chem. Geol. 418, 6–15 (2015).
Ulmer, P. & Trommsdorff, V. Serpentine stability to mantle depths and subduction-related magmatism. Science 268, 858-861 (1995).
Pawley, A. R. The pressure and temperature stability limits of lawsonite: implications for H2O recycling in subduction zones. Contrib. Mineral. Petr. 118, 99–108 (1994).
Schmidt, M. W. Lawsonite: upper pressure stability and formation of higher density hydrous phases. Am. Miner. 80, 1286–1292 (1995).
Sato, K., Katsura, T. & Ito, E. Phase relations of natural phlogopite with and without enstatite up to 8 GPa: implication for mantle metasomatism. Earth Planet. Sci. Lett. 146, 511–526 (1997).
Poli, S. & Schmidt, M. W. H2O transport and release in subduction zones: experimental constraints on basaltic and andesitic systems. J. Geophys. Res. 100, 22299–22314 (1995).
Okazaki, K. & Hirth, G. Dehydration of lawsonite could directly trigger earthquakes in subducting oceanic crust. Nature 530, 81–84 (2016).
Hyndman, R. D., Yamano, M. Y. & Oleskevich, D. A. The seismogenic zone of subduction thrust faults. Island Arc 6, 244–260 (1997).
Smyth, J. R. β-Mg2SiO4: A potential host for water in the mantle? Am. Miner. 72, 1051–1055 (1987).
Kohlstedt, D. L., Keppler, H. & Rubie, D. C. Solubility of water in the α, β and γ phases of (Mg,Fe)2SiO4. Contrib. Mineral. Petrol. 123, 345–357 (1996).
Schmandt, B., Jacobsen, S. D., Becker, T. W., Liu, Z. & Dueker, K. G. Dehydration melting at the top of the lower mantle. Science 344, 1265–1268 (2014).
Raleigh, C. & Paterson, M. Experimental deformation of serpentinite and its tectonic implications. J. Geophys. Res. 70, 3965–3985 (1965).
Jung, H., Green, H. W. II & Dobrzhinetskaya, L. F. Intermediate-depth earthquake faulting by dehydration embrittlement with negative volume change. Nature 428, 545–549 (2004).
Weaver, C. E. Clays, Muds, and Shales in Developments in Sedimentology Vol. 44, 1st edn (Elsevier, Amsterdam, 1989).
Windom, H. L. Lithogenous material in marine sediments. Chem. Oceanogr. 5, 103–135 (1976).
Syracuse, E. M., van Keken, P. E. & Abers, G. A. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 183, 73–90 (2010).
Laiglesia, A. Pressure-induced disorder in kaolinite. Clay Miner. 28, 311–319 (1993).
Johnston, C. T. et al. Novel pressure-induced phase transformations in hydrous layered materials. Geophys. Res. Lett. 29, 17-1–17-4 (2002).
Welch, M. D. & Crichton, W. A. Pressure-induced transformations in kaolinite. Am. Mineral. 95, 651–654 (2010).
Seoung, D., Lee, Y., Kao, C. C., Vogt, T. & Lee, Y. Super-hydrated zeolites: pressure-induced hydration in natrolites. Chem.-Eur. J. 19, 10876–10883 (2013).
Seoung, D., Lee, Y., Kao, C. C., Vogt, T. & Lee, Y. Two-step pressure-induced superhydration in small pore natrolite with divalent extra-framework cations. Chem. Mat. 27, 3874–3880 (2015).
You, S. J. et al. Pressure-induced water insertion in synthetic clays. Angew. Chem. Int. Ed. 52, 3891–3895 (2013).
Lee, Y., Vogt, T. & Hriljac, J. Pressure-induced migration of zeolitic water in laumontite. Phys. Chem. Miner. 31, 421–428 (2004).
White, C. L., Ruiz‐Salvador, A. R. & Lewis, D. W. Pressure-induced hydration effects in the zeolite laumontite. Angew. Chem. Int. Ed. 43, 469–472 (2004).
Simon, F. & Glatzel, G. Bemerkungen zur Schmelzdruckkurve. Z. Anorg. Allg. Chem. 178, 309–316 (1929).
Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 7, 1103–1112 (1939).
Wang, D., Yi, L., Huang, B. & Liu, C. High-temperature dehydration of talc: a kinetics study using in situ X-ray powder diffraction. Phase Transit. 88, 560–566 (2015).
Hancock, J. & Sharp, J. Method of comparing solid-state kinetic data and its application to the decomposition of kaolinite, brucite, and BaCO3. J. Am. Ceram. Soc. 55, 74–77 (1972).
Fumagalli, P., Stixrude, L., Poli, S. & Snyder, D. The 10Å phase: a high-pressure expandable sheet silicate stable during subduction of hydrated lithosphere. Earth Planet. Sci. Lett. 186, 125–141 (2001).
Welch, M. D., Pawley, A. R., Ashbrook, S. E., Mason, H. E. & Phillips, B. L. Si vacancies in the 10-Å phase. Am. Mineral. 91, 1707–1710 (2006).
Phillips, B. L., Mason, H. E. & Guggenheim, S. Hydrogen bonded silanols in the 10 Å phase: Evidence from NMR spectroscopy. Am. Mineral. 92, 1474–1485 (2007).
Pawley, A. R., Welch, M. D., Lennie, A. R. & Jones, R. L. Volume behavior of the 10 Å phase at high pressures and temperatures, with implications for H2O content. Am. Mineral. 95, 1671–1678 (2010).
Bish, D. L. & Vondreele, R. B. Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clay Clay Miner. 37, 289–296 (1989).
Welch, M. D., Montgomery, W., Balan, E. & Lerch, P. Insights into the high-pressure behavior of kaolinite from infrared spectroscopy and quantum-mechanical calculations. Phys. Chem. Miner. 39, 143–151 (2012).
Kodama, H. & Oinuma, K. Identification of kaolin minerals in the presence of chlorite by X-ray diffraction and infrared absorption spectra. Clay Clay Miner. 11, 236–249 (1963).
Wilson, M. J. Clay Mineralogy: Spectroscopic and Chemical Determinative Methods (Chapman & Hall, London, 1994).
Frost, R. L., Kloprogge, J. T., Thu, H. T. T. & Kristof, J. The effect of pressure on the intercalation of an ordered kaolinite. Am. Mineral. 83, 1182–1187 (1998).
Costanzo, P. M., Giese, R. F. & Lipsicas, M. Static and dynamic structure of water in hydrated kaolinites. 1. The static structure. Clay Clay Miner. 32, 419–428 (1984).
Daniels, P. & Wunder, B. Al3Si2O7(OH)3, phase Pi (formerly piezotite): Crystal structure of a synthetic high-pressure silicate rediscovered. Eur. J. Mineral. 8, 1283–1292 (1996).
Friedrich, A. et al. High-pressure properties of diaspore, AlO(OH). Phys. Chem. Mineral. 34, 145–157 (2007).
Kanzaki, M. Crystal structure of a new high-pressure polymorph of topaz-OH. Am. Mineral. 95, 1349–1352 (2010).
Ohtani, E. Water in the mantle. Elements 1, 25–30 (2005).
Karato, S. I., Paterson, M. S. & FitzGerald, J. D. Rheology of synthetic olivine aggregates: influence of grain size and water. J. Geophys. Res. 91, 8151–8176 (1986).
Iwamori, H. Transportation of H2O and melting in subduction zones. Earth Planet. Sci. Lett. 160, 65–80 (1998).
Inoue, T., Yurimoto, H. & Kudoh, Y. Hydrous modified spinel, Mg1.75SiH0.5O4—A new water reservoir in the mantle transition region. Geophys. Res. Lett. 22, 117–120 (1995).
Shieh, S. R., Mao, H.-k, Hemley, R. J. & Ming, L. C. Decomposition of phase D in the lower mantle and the fate of dense hydrous silicates in subducting slabs. Earth Planet. Sc. Lett. 159, 13–23 (1998).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800-kbar under quasihydrostatic conditions. J. Geophys. Res. 91, 4673–4676 (1986).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001).
Thompson, P., Cox, D. E. & Hastings, J. B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 20, 79–83 (1987).
Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 71, 809–824 (1947).
Comboni, D. et al. Pargasite at high pressure and temperature. Phys. Chem. Minerals https://doi.org/10.1007/s00269-017-0915-0 (2017).
Liermann, H.-P. et al. The Extreme Conditions Beamline P02.2 and the Extreme Conditions Science Infrastructure at PETRA III. J. Synchrotron Radiat. 22, 908–924 (2015).
Wang, Y. et al. Thermal equation of state of copper studied by high P–T synchrotron X-ray diffraction. Appl. Phys. Lett. 94, 071904 (2009)
Larson, A. C. & Von Dreele, R. B. GSAS—General Structure Analysis System (Los Alamos National Laboratory, 1986).
Sano, A., Ohtani, E., Kubo, T. & Funakoshi, K. In situ X-ray observation of decomposition of hydrous aluminum silicate AlSiO3OH and aluminum oxide hydroxide delta-AlOOH at high pressure and temperature. J. Phys. Chem. Solids. 65, 1547–1554 (2004).
Dollase, W. Correction of intensities for preferred orientation in powder diffractometry: application of the March model. J. Appl. Crystallogr. 19, 267–272 (1986).
Seagle, C. T., Heinz, D. L., Liu, Z. & Hemley, R. J. Synchrotron infrared reflectivity measurements of iron at high pressures. Appl. Opt. 48, 545–552 (2009).
Wunder, B. et al. Synthesis, stability, and properties of Al2SiO4(OH)2: A fully hydrated analogue of topaz. Am. Mineral. 78, 285–297 (1993).
Tsujimori, T., Sisson, V. B., Liou, J. G., Harlow, G. E. & Sorensen, S. S. Petrologic characterization of Guatemalan lawsonite eclogite: Eclogitization of subducted oceanic crust in a cold subduction zone. Geol. Soc. Am. S. 403, 147–168 (2006).
Acknowledgements
This work was supported by the Global Research Laboratory (NRF-2009-00408) and National Research Laboratory (NRF-2015R1A2A1A01007227) programs of the Korean Ministry of Science, ICT and Planning (MSIP). H.-K.M. was supported by NSF Grants EAR-1345112 and EAR-1447438. We also received surpport from NRF grants NRF-2016K1A4A3914691 and NRF-2016K1A3A7A09005244. Experiments using the synchrotrons were supported by the Collaborative Access Program of SSRL and general user programs of PAL, SSRF, ALS and APS. HPCAT operations are supported by DOE-NNSA under Award No. DE-NA0001974 and DOE-BES under Award No. DE-FG02-99ER45775, with partial instrumentation funding by NSF. The APS is supported by DOE-BES under Contract No. DE-AC02-06CH11357. The operation of the Infrared Lab of the NSLS-II at BNL was supported by COMPRES (EAR 1606856) and CDAC (DE-NA-0002006). NSLS-II is supported by DOE-BES under Contract No. DE-SC0012704. The ALS is support by DOE-BES under Contract No. DE-AC02-05CH11231. Part of this work was performed under the auspices of the US DOE by LLNL under Contract DE-AC52-07NA27344. Parts of this research were carried out at PETRA III at DESY, a member of the Helmholtz Association (HGF).
Author information
Authors and Affiliations
Contributions
H. H. contributed to the experiments and data analysis with the help from D.S., Z.L., H.-P.L. and H.C. Y.L. designed the research, discussed the results with T.V., C.-C.K. and H.-K.M. and worked on the manuscript with all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary experimental data and discussion
Rights and permissions
About this article
Cite this article
Hwang, H., Seoung, D., Lee, Y. et al. A role for subducted super-hydrated kaolinite in Earth’s deep water cycle. Nature Geosci 10, 947–953 (2017). https://doi.org/10.1038/s41561-017-0008-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-017-0008-1
This article is cited by
-
Structure evolution of hydroxyapophyllite-(K) under high pressure
Physics and Chemistry of Minerals (2024)
-
Influence of pH on the Hydrothermal Synthesis of Al-Substituted Smectites (Saponite, Beidellite, and Nontronite)
Clays and Clay Minerals (2023)
-
2M1 phlogopite–muscovite series minerals at increasing pressure to 9 GPa. I Atomic volumes and compressibilities
Physics and Chemistry of Minerals (2023)
-
Super-hydration and reduction of manganese oxide minerals at shallow terrestrial depths
Nature Communications (2022)
-
Development and Characterization of Sintered Zeolite and Zeolite-Kaolin Wick Structure for Thermosiphon Heat-Pipe Application
Clays and Clay Minerals (2022)