Abstract
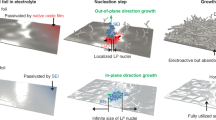
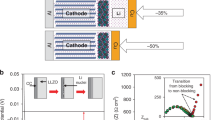
Controlling the nucleation and growth of lithium metal is essential for realizing fast-charging batteries. Here we report the growth of single-crystalline seeds that results in the deposition of dense lithium, even at high current densities. Contrary to the widely accepted practice of using a lithiophilic surface to achieve dendrite-free deposition, we employ a lithiophobic surface made of a nanocomposite of LiF and Fe to deposit hexagonal crystals, which induce subsequent dense lithium deposition. The nanocomposites have uniform Fe sites for nucleation while LiF enables rapid lithium transport. A cell using a 3 mAh cm−2 LiNi0.8Co0.1Mn0.1O2 (LiNMC811) cathode, onefold excess of lithium and 3 g Ah−1 electrolyte cycles at a 1 C rate for more than 130 cycles with 80% capacity retention, a 550% improvement over the baseline cells. Our findings advance the understanding of lithium nucleation and pave the way for realizing high-energy, fast-charging Li-metal batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information. Source data are provided with this paper.
References
Kim, M. S. et al. Suspension electrolyte with modified Li+ solvation environment for lithium-metal batteries. Nat. Mater. 21, 445–454 (2022).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium-metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Liu, Y. et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium-metal batteries. Science 375, 739–745 (2022).
Holoubek, J. et al. Electrolyte design implications of ion-pairing in low-temperature Li-metal batteries. Energy Environ. Sci. 15, 1647–1658 (2022).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li-metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Niu, C. et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium-metal batteries. Nat. Energy 6, 723–732 (2021).
Liu, H. et al. Ultrahigh Coulombic efficiency electrolyte enables Li||SPAN batteries with superior cycling performance. Mater. Today 42, 17–28 (2021).
Holoubek, J. et al. Tailoring electrolyte solvation for Li-metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Liu, H. et al. An anode-free Li-metal cell with replenishable Li designed for long cycle life. Energy Storage Mater. 36, 251–256 (2021).
Hu, A. et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium-metal anode. Energy Environ. Sci. 14, 4115–4124 (2021).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium-metal batteries. Nat. Energy 5, 526–533 (2020).
Louli, A. J. et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020).
Zhou, H., Yu, S., Liu, H. & Liu, P. Protective coatings for lithium-metal anodes: recent progress and future perspectives. J. Power Sources 450, 227632 (2020).
Gao, Y. et al. Low-temperature and high-rate-charging lithium-metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat. Energy 5, 534–542 (2020).
Weber, R. et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019).
Niu, C. et al. High-energy lithium-metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 4, 551–559 (2019).
Xiang, Y. et al. Quantitatively analyzing the failure processes of rechargeable Li-metal batteries. Sci. Adv. 7, abj3423 (2021).
Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Zhao, Q. et al. On the crystallography and reversibility of lithium electrodeposits at ultrahigh capacity. Nat. Commun. 12, 6034 (2021).
Chen, X., Zhao, B., Yan, C. & Zhang, Q. Review on Li deposition in working batteries: from nucleation to early growth. Adv. Mater. 33, 2004128 (2021).
Zhang, Y. et al. Towards better Li-metal anodes: challenges and strategies. Mater. Today 33, 56–74 (2020).
Zheng, J. et al. Regulating electrodeposition morphology of lithium: towards commercially relevant secondary Li-metal batteries. Chem. Soc. Rev. 49, 2701–2750 (2020).
Fang, C., Wang, X. & Meng, Y. S. Key issues hindering a practical lithium-metal anode. Trends Chem. 1, 152–158 (2019).
Fang, C. et al. Quantifying inactive lithium in lithium-metal batteries. Nature 572, 511–515 (2019).
Lv, D. et al. Failure mechanism for fast-charged lithium-metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Wu, Z. et al. The role of ion transport in the failure of high-areal-capacity Li-metal batteries. ACS Energy Lett. 7, 2701–2710 (2022).
Zhang, J. G., Xu, W., Xiao, J., Cao, X. & Liu, J. Lithium-metal anodes with nonaqueous electrolytes. Chem. Rev. 120, 13312–13348 (2020).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium-metal batteries. Nat. Energy 6, 987–994 (2021).
Chen, T. et al. Ultra-low concentration electrolyte enabling LiF-rich SEI and dense plating/stripping processes for lithium-metal batteries. Adv. Sci. 9, 2203216 (2022).
Sun, H. H. et al. In situ formation of a multicomponent inorganic-rich SEI layer provides a fast-charging and high-specific-energy Li-metal battery. J. Mater. Chem. A 7, 17782–17789 (2019).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium-metal batteries. Nat. Energy 4, 180–186 (2019).
Sun, J. et al. Li deposition mechanism on Si and Cu substrates in the carbonate electrolyte. Energy Environ. Sci. https://doi.org/10.1039/d2ee01833k (2022).
Cui, S. et al. Large-scale modification of commercial copper foil with lithiophilic metal layer for Li-metal battery. Small 16, 1905620 (2020).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium-metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Lee, D. et al. Copper nitride nanowires printed Li with stable cycling for Li-metal batteries in carbonate electrolytes. Adv. Mater. 32, 1905573 (2020).
Niu, C. et al. Self-smoothing anode for achieving high-energy lithium-metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019).
Li, Z. et al. Gradient nano-recipes to guide lithium deposition in a tunable reservoir for anode-free batteries. Energy Storage Mater. 45, 40–47 (2022).
Yang, C. et al. Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium-metal anode. Adv. Mater. 29, 1702714 (2017).
Lu, Y. et al. Spatially controlled lithium deposition on silver-nanocrystals-decorated TiO2 nanotube arrays enabling ultrastable lithium-metal anode. Adv. Funct. Mater. 31, 2009605 (2021).
Hao, F., Verma, A. & Mukherjee, P. P. Mesoscale complexations in lithium electrodeposition. ACS Appl. Mater. Interfaces 10, 26320–26327 (2018).
Coaty, C., Zhou, H., Liu, H. & Liu, P. A scalable synthesis pathway to nanoporous metal structures. ACS Nano 12, 432–440 (2018).
Zhang, X.-Q. et al. Columnar lithium-metal anodes. Angew. Chem. Int. Ed. 56, 14207–14211 (2017).
Qian, J. et al. Dendrite-free Li deposition using trace-amounts of water as an electrolyte additive. Nano Energy 15, 135–144 (2015).
Saifullah, M. S. M., Botton, G. A., Boothroyd, C. B. & Humphreys, C. J. Electron energy loss spectroscopy studies of the amorphous to crystalline transition in FeF3. J. Appl. Phys. 86, 2499–2504 (1999).
Li, T., Li, L., Cao, Y. L., Ai, X. P. & Yang, H. X. Reversible three-electron redox behaviors of FeF3 nanocrystals as high-capacity cathode-active materials for Li-ion batteries. J. Phys. Chem. C 114, 3190–3195 (2010).
Wang, J. et al. Fundamental study on the wetting property of liquid lithium. Energy Storage Mater. 14, 345–350 (2018).
Shi, L., Bak, S., Shadike, Z. & Wang, C. Reaction heterogeneity in practical high-energy lithium–sulfur pouch cells. Energy Environ. Sci. 13, 3620–3632 (2020).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 358, 506–510 (2017).
Yamakawa, N., Jiang, M., Key, B. & Grey, C. P. Identifying the local structures formed during lithiation of the conversion material, iron fluoride, in a Li ion battery: a solid-state NMR, X-ray diffraction, and pair distribution function analysis study. J. Am. Chem. Soc. 131, 10525–10536 (2009).
Zheng, H. et al. Grain boundary properties of elemental metals. Acta Mater. 186, 40–49 (2020).
Tran, R. et al. Surface energies of elemental crystals. Sci. Data 3, 160080 (2016).
Tran, R. et al. Anisotropic work function of elemental crystals. Surf. Sci. 687, 48–55 (2019).
Shi, F. et al. Strong texturing of lithium-metal in batteries. Proc. Natl Acad. Sci. USA 114, 12138–12143 (2017).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. Accurate determination of Coulombic efficiency for lithium-metal anodes and lithium-metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Li, Q. et al. Homogeneous interface conductivity for lithium dendrite-free anode. ACS Energy Lett. 3, 2259–2266 (2018).
Heim, F., Kreher, T. & Birke, K. P. The influence of micro-structured anode current collectors in combination with highly concentrated electrolyte on the Coulombic efficiency of in situ deposited Li-metal electrodes with different counter electrodes. Batteries 6, 20–30 (2020).
Wang, J. et al. Improving cyclability of Li-metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019).
Biswal, P. et al. The early-stage growth and reversibility of Li electrodeposition in Br-rich electrolytes. Proc. Natl Acad. Sci. USA 118, e2012071118 (2021).
Chen, S. et al. Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries. Joule 3, 1094–1105 (2019).
Kim, M. S. et al. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium-metal batteries. Nat. Energy 3, 889–898 (2018).
Chen, H. et al. Tortuosity effects in lithium-metal host anodes. Joule 4, 938–952 (2020).
Acknowledgements
The work was supported by the Office of Vehicle Technologies of the US Department of Energy through the Advanced Battery Materials Research Program (Battery500 Consortium) under contract no. DE-EE0007764. Work done by C.W. and H.L.X. at UCI was supported by the Materials Science and Engineering Divisions, Office of Basic Energy Sciences of the US Department of Energy, under award no. DE-SC0021204. Part of the work used the UCSD-MTI Battery Fabrication Facility and the UCSD-Arbin Battery Testing Facility. Part of the characterization was performed at the San Diego Nanotechnology Infrastructure of UCSD, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS-1542148). This research used the TEM facility of the Center for Functional Nanomaterials, which is a US Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under contract no. DE-SC0012704.
Author information
Authors and Affiliations
Contributions
Z.W. and P.L. conceived the idea. P.L. and H.L. directed the project. Z.W. and Z.H. performed the electrochemical experiments and SEM characterizations. S.W. performed the FIB-SEM analysis. S.Y. carried out the XPS characterization. X.X. developed the method for the thermal evaporation of iron fluoride. J.H. provided input on data interpretation and manuscript preparation. Q.M. helped with the contact angle measurement. C.W. and H.L.X. performed the TEM measurement and analysis. Z.W., C.W., Z.H., H.L., J.H., H.L.X. and P.L. co-wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Maximilian Becker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27 and Table 1.
Source data
Source Data Fig. 4
Statistical data for Fig. 4c,d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Z., Wang, C., Hui, Z. et al. Growing single-crystalline seeds on lithiophobic substrates to enable fast-charging lithium-metal batteries. Nat Energy 8, 340–350 (2023). https://doi.org/10.1038/s41560-023-01202-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01202-1
This article is cited by
-
Hierarchical Li electrochemistry using alloy-type anode for high-energy-density Li metal batteries
Nature Communications (2024)
-
Making the deposition surface lithiophobic
Nature Energy (2023)
-
SEI growth on Lithium metal anodes in solid-state batteries quantified with coulometric titration time analysis
Nature Communications (2023)
-
High-performance lithium-ion batteries based on polymer/graphene hybrid cathode material
Science China Chemistry (2023)
-
Conversion reaction lithium metal batteries
Nano Research (2023)