Abstract
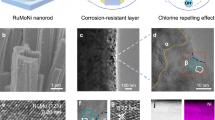
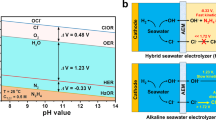
The use of vast amounts of high-purity water for hydrogen production may aggravate the shortage of freshwater resources. Seawater is abundant but must be desalinated before use in typical proton exchange membrane (PEM) electrolysers. Here we report direct electrolysis of real seawater that has not been alkalised nor acidified, achieving long-term stability exceeding 100 h at 500 mA cm−2 and similar performance to a typical PEM electrolyser operating in high-purity water. This is achieved by introducing a Lewis acid layer (for example, Cr2O3) on transition metal oxide catalysts to dynamically split water molecules and capture hydroxyl anions. Such in situ generated local alkalinity facilitates the kinetics of both electrode reactions and avoids chloride attack and precipitate formation on the electrodes. A flow-type natural seawater electrolyser with Lewis acid-modified electrodes (Cr2O3–CoOx) exhibits the industrially required current density of 1.0 A cm−2 at 1.87 V and 60 °C.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets analysed and generated during the current study are included in the paper and Supplementary Information. Source data are provided with this paper.
References
Turner, J. Sustainable hydrogen production. Science 305, 972–974 (2004).
Rashid, M. R., Al Mesfer, M. K., Naseem, H. & Mohd, D. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 4, 2249–8958 (2015).
Stiber, S. et al. A high-performance, durable and low-cost proton exchange membrane electrolyser with stainless steel components. Energy Environ. Sci. 15, 109–122 (2022).
Kuang, Y. et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl Acad. Sci. USA 116, 6624–6629 (2019).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
Dionigi, F., Reier, T., Pawolek, Z., Gliech, M. & Strasser, P. Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 9, 962–972 (2016).
Dresp, S. et al. Molecular understanding of the impact of saline contaminants and alkaline pH on NiFe layered double hydroxide oxygen evolution catalysts. ACS Catal. 11, 6800–6809 (2021).
Hausmann, J. N., Schlӧgl, R., Menezes, P. W. & Driess, M. Is direct seawater splitting economically meaningful? Energy Environ. Sci. 14, 3679–3685 (2021).
Khan, M. A. et al. Seawater electrolysis for hydrogen production: a solution looking for a problem? Energy Environ. Sci. 14, 4831–4839 (2021).
Dresp, S., Dionigi, F., Klingenhof, M. & Strasser, P. Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019).
Kana, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Anantharaj, S. & Aravindan, V. Developments and perspectives in 3D transition‐metal‐based electrocatalysts for neutral and near‐neutral water electrolysis. Adv. Energy Mater. 10, 1902666 (2020).
Kapp, E. M. The precipitation of calcium and magnesium from seawater by sodium hydroxide. Biol. Bull. 55, 453–458 (1928).
Kirk, D. W. & Ledas, A. E. Precipitate formation during sea water electrolysis. Int. J. Hydrog. Energy 7, 925–932 (1982).
Li, P., Jin, Z. & Xiao, D. A one-step synthesis of Co–P–B/rGO at room temperature with synergistically enhanced electrocatalytic activity in neutral solution. J. Mater. Chem. A 2, 18420–18427 (2014).
Hsu, S. H. et al. An earth-abundant catalyst-based seawater photoelectrolysis system with 17.9% solar-to-hydrogen efficiency. Adv. Mater. 30, 1707261 (2018).
Yu, L. et al. Hydrogen generation from seawater electrolysis over a sandwich-like NiCoN|NixP|NiCoN micro-sheet array catalyst. ACS Energy Lett. 5, 2681–2689 (2020).
Lu, X. et al. A sea-change: manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 11, 1898–1910 (2018).
Yu, L. et al. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 13, 3439–3446 (2020).
Wu, L. et al. Heterogeneous bimetallic phosphide Ni2P–Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 31, 2006484 (2021).
Gao, X. et al. Karst landform-featured monolithic electrode for water electrolysis in neutral media. Energy Environ. Sci. 13, 174–182 (2020).
Zhao, Y. et al. Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 8, 1801926 (2018).
Park, J. E. et al. High-performance proton-exchange membrane water electrolysis using a sulfonated poly (arylene ether sulfone) membrane and ionomer. J. Membr. Sci. 620, 118871 (2021).
Lindquist, G. A., Xu, Q., Oener, S. Z. & Boettcher, S. W. Membrane electrolyzers for impure-water splitting. Joule 4, 2549–2561 (2020).
Surendranath, Y., Matthew, W. & Daniel, G. Mechanistic studies of the oxygen evolution reaction by a cobalt–phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Pearson, R. G. Hard and soft acids and bases, HSAB, part 1: fundamental principles. J. Chem. Educ. 45, 581–587 (1968).
Magaz, G. E., Rodenas, L. G., Morando, P. J. & Blesa, M. A. Electrokinetic behaviour and interaction with oxalic acid of different hydrous chromium(III) oxides. Croat. Chem. Acta 71, 917–927 (1998).
Yu, L. et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 10, 5106 (2019).
Li, Y. et al. Sandwich structured Ni3S2–MoS2–Ni3S2@Ni foam electrode as a stable bifunctional electrocatalyst for highly sustained overall seawater splitting. Electrochim. Acta 390, 138833 (2021).
Wang, C. et al. Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B 291, 120071–120079 (2021).
Rodríguez, R., Blesa, M. A. & Regazzonil, A. E. Surface complexation at the TiO2(anatase)/aqueous solution interface: chemisorption of catechol. J. Colloid Interface Sci. 177, 121–131 (1996).
Yokoyama, Y. et al. In situ local pH measurements with hydrated iridium oxide ring electrodes in neutral pH aqueous solutions. Chem. Lett. 49, 195–198 (2020).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2001).
Kunimatsu, K., Yoda, T., Tryk, D. A., Uchida, H. & Watanabe, M. In situ ATR-FTIR study of oxygen reduction at the Pt/Nafion interface. Phys. Chem. Chem. Phys. 12, 621–629 (2010).
Zhu, S., Qin, X., Yao, Y. & Shao, M. pH-Dependent hydrogen and water binding energies on platinum surfaces as directly probed through surface-enhanced infrared absorption spectroscopy. J. Am. Chem. Soc. 142, 8748–8754 (2020).
Deng, W. et al. Crucial role of surface hydroxyls on the activity and stability in electrochemical CO2 reduction. J. Am. Chem. Soc. 141, 2911–2915 (2019).
Kitano, S. et al. Heterointerface created on Au cluster‐loaded unilamellar hydroxide electrocatalysts as a highly active site for the oxygen evolution reaction. Adv. Mater. 37, 2110552 (2022).
Yang, X. et al. Understanding the pH dependence of underpotential deposited hydrogen on platinum. Angew. Chem. 131, 17882–17887 (2019).
Ackermann, T. Hydration of H+ and OH– ions in water from heat capacity measurements. Discuss. Faraday Soc. 24, 180–193 (1957).
Wang, X., Xu, C., Jaroniec, M., Zheng, Y. & Qiao, S. Z. Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019).
Yan, L. et al. Electronic modulation of cobalt phosphide nanosheet arrays via copper doping for highly efficient neutral-pH overall water splitting. Appl. Catal. B 265, 118555–118566 (2020).
Liu, L. et al. The rational design of Cu2−xSe@(Co, Cu)Se2 core–shell structures as bifunctional electrocatalysts for neutral-pH overall water splitting. Nanoscale 13, 1134–1143 (2021).
Ling, T. et al. Activating cobalt(II) oxide nanorods for efficient electrocatalysis by strain engineering. Nat. Commun. 8, 1509 (2017).
Ling, T. et al. Atomic-level structure engineering of metal oxides for high-rate oxygen intercalation pseudocapacitance. Sci. Adv. 4, eaau6261 (2018).
Kim, B. et al. Vertical-crystalline Fe-doped β-Ni oxyhydroxides for highly active and stable oxygen evolution reaction. Matter 4, 3585–3604 (2021).
Kuai, C. et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts. Nat. Catal. 4, 743–753 (2020).
Zheng, X. et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 10, 149–154 (2017).
Yamanaka, K. Anodically electrodeposited iridium oxide films from alkaline solutions for electrochromic display devices. Jpn. J. Appl. Phys. 28, 632–637 (1989).
Steegstra, P. & Ahlberg, E. In situ pH measurements with hydrous iridium oxide in a rotating ring disc configuration. J. Electroanal. Chem. 685, 1–7 (2012).
Albery, W. J. & Calvo, E. J. Ring–disc electrodes. Part 21.—pH measurement with the ring. J. Chem. Soc. Faraday Trans. 1 79, 2583–2596 (1983).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Dudarev, S. L. et al. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Li, A. et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat. Catal. 5, 109–118 (2022).
Chan, K. & Nørskov, J. K. Electrochemical barriers made simple. J. Phys. Chem. Lett. 6, 2663–2668 (2015).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by the Natural Science Foundation of China (52071231 and 51722103) and the Natural Science Foundation of Tianjin city (19JCJQJC61900). Y.Z. acknowledges funding from the Australian Research Council (DP190103472 and FT200100062). S.-Z.Q. acknowledges funding from the Australian Research Council (FL170100154 and DP220102596). Calculations were performed on TianHe-1A at the National Supercomputer Center, Tianjin. We thank Weihua Wang from Nankai University for constructive suggestions.
Author information
Authors and Affiliations
Contributions
T.L., Y.Z. and S.-Z.Q. conceived the project and designed the experiments. J.G. and K.D. performed the experiments. Z.H., C.Z. and T.L. carried out the DFT calculations. J.M. carried out the TEM and HAADF-STEM characterizations. T.L., Y.Z. and J.G. wrote the manuscript. S.-Z.Q., T.L. and M.J. reviewed and corrected the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Wen-Feng Lin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–9, Figs. 1–56 and Tables 1 and 2.
Supplementary Data
Source data for Supplementary Fig. 36c.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Zheng, Y., Hu, Z. et al. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat Energy 8, 264–272 (2023). https://doi.org/10.1038/s41560-023-01195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01195-x
This article is cited by
-
Heterojunction interface engineering for stable and effective porous Fe3O4-ZnO nanocomposites: a highly efficient synergistic catalyst for oxygen evolution reaction
Chemical Papers (2024)
-
Self-adaptive amorphous CoOxCly electrocatalyst for sustainable chlorine evolution in acidic brine
Nature Communications (2023)
-
Advances on Axial Coordination Design of Single-Atom Catalysts for Energy Electrocatalysis: A Review
Nano-Micro Letters (2023)