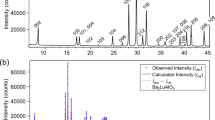
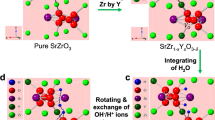
Abstract
Solid oxide ionic conductors are employed in a wide range of energy-conversion applications, such as electrolytes in fuel cells. Typically, conventional ionic conductors based on metal oxides require elevated temperatures above approximately 500 °C to activate ionic transport, but the ability to operate at lower temperature could avoid mechanical instability and operating complexities. Here we report a solid oxide proton conductor, HSrCoO2.5, which shows unusually high proton conductivity between 40 °C and 140 °C. The proton conductivity was between 0.028 S cm−1 to 0.33 S cm−1 in this temperature range, with an ionic activation energy of approximately 0.27 eV. Combining experimental results and first-principles calculations, we attribute these intriguing properties to the high proton concentration and the well-ordered oxygen vacancy channels granted by the hydrogen-intercalated brownmillerite crystalline structure. Our results open the possibility of using solid oxide materials as the proton-conducting electrolytes in low-temperature devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and Supplementary Information files. Source data in the Main Text and Supplementary Information are provided with this paper. Source data are provided with this paper.
Change history
07 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41560-023-01349-x
References
Goodenough, J. B. Oxide-ion electrolytes. Annu. Rev. Mater. Res. 33, 91–128 (2003).
Steele, B. C. H. & Heinzel, A. Materials for fuel-cell technologies. Nature 414, 345–352 (2001).
Malavasi, L., Fisher, C. A. J. & Islam, M. S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem. Soc. Rev. 39, 4370–4387 (2010).
Shao, Z. P. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170 (2004).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Garcia-Barriocanal, J. et al. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 321, 676–680 (2008).
Shi, Y. N., Bork, A. H., Schweiger, S. & Rupp, J. L. M. The effect of mechanical twisting on oxygen ionic transport in solid-state energy conversion membranes. Nat. Mater. 4, 721–727 (2015).
Malerød-Fjeld, H. et al. Thermo-electrochemical production of compressed hydrogen from methane with near-zero energy loss. Nat. Energy 2, 923–931 (2017).
Maier, J. Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4, 805–815 (2005).
Kreuer, K. D. Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003).
Ishihara, T. (ed.) Perovskite Oxide for Solid Oxide Fuel Cells (Springer, 2009).
Fabbri, E., Pergolesi, D. & Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: a critical review. Chem. Soc. Rev. 39, 4355–4369 (2010).
Pergolesi, D. et al. High proton conduction in grain-boundary-free yttrium-doped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 9, 846–852 (2010).
Yamazaki, Y. et al. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 12, 647–651 (2013).
Zhou, Y. et al. Strongly correlated perovskite fuel cells. Nature 534, 231–234 (2016).
Zhang, G. B. & Smyth, D. M. Protonic conduction in Ba2In2O5. Solid State Ion. 82, 153–160 (1995).
Fisher, C. A. J. & Islam, M. S. Defect, protons and conductivity in brownmillerite-structured Ba2In2O5. Solid State Ion. 118, 355–363 (1999).
Kreuer, K. D. Proton conductivity: materials and applications. Chem. Mater. 8, 610–641 (1996).
Münch, W., Kreuer, K. D., Seifert, G. & Maier, J. Proton diffusion in perovskites: comparison between BaCeO3, BaZrO3, SrTiO3, and CaTiO3 using quantum molecular dynamics. Solid State Ion. 136/137, 183–189 (2000).
Muñoz, A. et al. Crystallographic and magnetic structure of SrCoO2.5 brownmillerite: neutron study coupled with band-structure calculations. Phys. Rev. B 78, 054404 (2008).
Lu, N. P. et al. Electric–field control of tri-state phase transformation with a selective dual-ion switch. Nature 546, 124–128 (2017).
Macdonald, J. R. Simplified impedance/frequency-response results for intrinsically conduting solids and liquids. J. Chem. Phys. 61, 3977–3996 (1974).
Haile, S. M., Boysen, D. A., Chisholm, C. R. I. & Merle, R. B. Solid acids as fuel cell electrolytes. Nature 410, 910–913 (2001).
Ishihara, T., Shibayama, T., Honda, M., Nishiguchi, H. & Takita, Y. Intermediate temperature solid oxide fuel cells using LaGaO3 electrolyte II. improvement of oxide ion conductivity and power density by doping Fe for Ga site of LaGaO3. J. Electrochem. Soc. 147, 1332–1337 (2000).
Esposito, V. & Traversa, E. Design of electroceramics for solid oxides fuel cell applications: playing with ceria. J. Am. Ceram. Soc. 91, 1037–1051 (2008).
Fabbri, E., D’Epifanio, A., Di Bartolomeo, E., Licoccia, S. & Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−xZrx)Y0.2O3−δ protonic conductors for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ion. 179, 558–564 (2008).
Xu, H., Tao, S. S. & Jiang, D. L. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 15, 722–726 (2016).
Hurd, J. A. et al. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework. Nat. Chem. 1, 705–710 (2009).
He, X. F., Zhu, Y. Z. & Mo, Y. F. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
O’Hayre, R. et al. Fuel Cell Fundamentals (John Wiley & Sons, 2016).
Fukui, K. et al. Characteristic fast H− ion conduction in oxygen-substituted lanthanum hydride. Nat. Commun. 10, 2578 (2019).
Kobayashi, G. et al. Pure H– conduction in oxyhydrides. Science 351, 1314 (2016).
Acknowledgements
We thank Z.-P. Shao, Y. Zhou and C.-C. Duan for helpful discussions and critical reading of the manuscript and Y. Shi and T. Liu for experimental assistance. This study was financially supported by the Basic Science Center Project of NFSC (grant number 51788104, P.Y. and C.-W.N.), the Ministry of Science and Technology of China (grant number 2021YFE0107900, P.Y.), the Beijing Nature Science Foundation (grant number Z200007, P.Y.), the National Key R&D Program of China (grant number 2021YFA1400100, S.Z., and 2021YFA1400300, P.Y.), the National Natural Science Foundation of China (grant nos. 52025024 and 51872155, P.Y.), the Beijing Advanced Innovation Center for Future Chip (ICFC, P.Y. and S.Z.) and Major Science and Technology Project of Precious Metal Materials Genome Engineering in Yunnan Province (grant number 2019ZE001-1, J.W.). N.L. acknowledges support from the National Natural Science Foundation of China (grant number 11974401), CAS Project for Young Scientists in Basic Research (grant number YSBR-047) and the Strategic Priority Research Program of Chinese Academy of Sciences of China (grant number XDB33000000).
Author information
Authors and Affiliations
Contributions
P.Y. conceived the project and designed the experiments. N.L., Yongshun.Wu., S.L., X.C., M.Z., Z.L. and Jianbing.Zhang. fabricated the thin films. N.L., Y.Wang. and H.L. carried out ILG, structural, optical and compositional characterization. N.L., C.L. and X.L. performed the impedance spectroscopy, d.c. measurement and dual-chamber fuel cell characterization and test, with help from H.L., D.Z., X.C., S.Y., M.W. and J.M. Z.Z, S.Q., B.Z. and J.R. performed the theoretical analysis and calculations, under the supervision of J.W. Q.H., J.G. and K.D. performed the soft X-ray absorption measurements. Jingzhao.Zhang., S.C.T., J.Zhu., Yang.Wu., S.Z., Y.T. and C.-W.N. discussed the results. N.L. and P.Y. wrote the manuscript, and all authors discussed results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Shriram Ramanathan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Notes 1–6 and Tables 1 and 2.
Supplementary Data
Source data for the supplementary figures.
Source data
Source Data Fig. 2
Source data for the plots in a and b.
Source Data Fig. 3
Source data for the plots in e and f.
Source Data Fig. 4
Source data for the plot in a.
Source Data Fig. 5
Source data for the plots in a–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, N., Zhang, Z., Wang, Y. et al. Enhanced low-temperature proton conductivity in hydrogen-intercalated brownmillerite oxide. Nat Energy 7, 1208–1216 (2022). https://doi.org/10.1038/s41560-022-01166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01166-8
This article is cited by
-
Nanoscale multistate resistive switching in WO3 through scanning probe induced proton evolution
Nature Communications (2023)
-
Tunable ferroelectricity in oxygen-deficient perovskites with Grenier structure
npj Computational Materials (2023)
-
Cool proton conductors
Nature Energy (2022)