Abstract
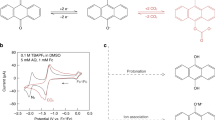
Carbon capture is considered a critical means for climate change mitigation. However, conventional wet chemical scrubbing utilizing sp3 amines suffers from high energy consumption, corrosion and sorbent degradation, motivating the search for more efficient carbon dioxide separation strategies. Here, we demonstrate a library of redox-tunable Lewis bases with sp2-nitrogen centres that can reversibly capture and release carbon dioxide through an electrochemical cycle. The mechanism of the carbon capture process is elucidated via a combined experimental and computational approach. We show that the properties of these Lewis base sorbents can be fine-tuned via molecular design and electrolyte engineering. Moreover, we identify a bifunctional azopyridine base that holds promise for electrochemically mediated carbon capture, exhibiting >85% capacity utilization efficiency over cycling in a flow system under 15% carbon dioxide with 5% oxygen. This work broadens the structural scope of redox-active carbon dioxide sorbents and provides design guidelines on molecules with tunable basicity under electrochemical conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated or analysed during this study are included in the published article and its Supplementary Information file. Source data are provided with this paper.
References
IPCC Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) (WMO, 2018).
Chu, S. Carbon capture and sequestration. Science 325, 1599 (2009).
Haszeldine, R. S. Carbon capture and storage: how green can black be? Science 325, 1647–1652 (2009).
Bui, M. et al. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176 (2018).
Shi, X. et al. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 59, 6984–7006 (2020).
Sanz-Pérez, E. S., Murdock, C. R., Didas, S. A. & Jones, C. W. Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876 (2016).
Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652–1654 (2009).
Said, R. B., Kolle, J. M., Essalah, K., Tangour, B. & Sayari, A. A unified approach to CO2–amine reaction mechanisms. ACS Omega 5, 26125–26133 (2020).
Davis, J. & Rochelle, G. Thermal degradation of monoethanolamine at stripper conditions. Energy Procedia 1, 327–333 (2009).
Rochelle, G. T. in Absorption-Based Post-combustion Capture of Carbon Dioxide 35–67 (Woodhead Publishing, 2016).
Vasudevan, S. et al. Energy penalty estimates for CO2 capture: comparison between fuel types and capture-combustion modes. Energy 103, 709–714 (2016).
Zheng, R. F. et al. A single-component water-lean post-combustion CO2 capture solvent with exceptionally low operational heat and total costs of capture—comprehensive experimental and theoretical evaluation. Energy Environ. Sci. 13, 4106–4113 (2020).
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal–organic frameworks. Nature 519, 303–308 (2015).
Park, Y., Lin, K.-Y. A., Park, A.-H. A. & Petit, C. Recent advances in anhydrous solvents for CO2 capture: ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front. Energy Res. 3, 42 (2015).
Halliday, C. & Hatton, T. A. Sorbents for the capture of CO2 and other acid gases: a review. Ind. Eng. Chem. Res. 60, 9313–9346 (2021).
Rheinhardt, J. H., Singh, P., Tarakeshwar, P. & Buttry, D. A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2, 454–461 (2017).
Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. ACS Catal. 10, 13058–13074 (2020).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Stern, M. C., Simeon, F., Herzog, H. & Hatton, T. A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 6, 2505–2517 (2013).
Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 12, 3530–3547 (2019).
Liu, Y. et al. Electrochemically mediated gating membrane with dynamically controllable gas transport. Sci. Adv. 6, eabc1741 (2020).
Liu, Y., Ye, H.-Z., Diederichsen, K. M., Van Voorhis, T. & Hatton, T. A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 11, 2278 (2020).
Diederichsen, K. M., Liu, Y., Ozbek, N., Seo, H. & Hatton, T. A. Toward solvent-free continuous-flow electrochemically mediated carbon capture with high-concentration liquid quinone chemistry. Joule 6, 221–239 (2022).
Seo, H., Rahimi, M. & Hatton, T. A. Electrochemical carbon dioxide capture and release with a redox-active amine. J. Am. Chem. Soc. 144, 2164–2170 (2022).
Mizen, M. B. & Wrighton, M. S. Reductive addition of CO2 to 9,10-phenanthrenequinone. J. Electrochem. Soc. 136, 941–946 (1989).
Scovazzo, P., Poshusta, J., DuBois, D., Koval, C. & Noble, R. Electrochemical separation and concentration of <1% carbon dioxide from nitrogen. J. Electrochem. Soc. 150, D91 (2003).
Simpson, T. C. & Durand, R. R. Reactivity of carbon dioxide with quinones. Electrochim. Acta 35, 1399–1403 (1990).
Simeon, F. et al. Electrochemical and molecular assessment of quinones as CO2-binding redox molecules for carbon capture. J. Phys. Chem. C 126, 1389–1399 (2022).
Singh, P. et al. Electrochemical capture and release of carbon dioxide using a disulfide–thiocarbonate redox cycle. J. Am. Chem. Soc. 139, 1033–1036 (2017).
Ranjan, R. et al. Reversible electrochemical trapping of carbon dioxide using 4,4′-bipyridine that does not require thermal activation. J. Phys. Chem. Lett. 6, 4943–4946 (2015).
Assary, R. S., Brushett, F. R. & Curtiss, L. A. Reduction potential predictions of some aromatic nitrogen-containing molecules. RSC Adv. 4, 57442–57451 (2014).
Vasudevan, D. & Wendt, H. Electroreduction of oxygen in aprotic media. J. Electroanal. Chem. 392, 69–74 (1995).
Zhuang, Z. et al. Spectroscopy of 4,4′-azopyridine by density functional theory and surface-enhanced Raman scattering. J. Mol. Struct. 794, 77–82 (2006).
Wei, X. et al. Towards high-performance nonaqueous redox flow electrolyte via ionic modification of active species. Adv. Energy Mater. 5, 1400678 (2015).
Milshtein, J. D. et al. Towards low resistance nonaqueous redox flow batteries. J. Electrochem. Soc. 164, A2487–A2499 (2017).
Yuan, J. et al. Membranes in non-aqueous redox flow battery: a review. J. Power Sources 500, 229983 (2021).
IPCC Special Report on Carbon Dioxide Capture and Storage (eds Metz, B. et al.) (Cambridge Univ. Press, 2005).
Winkler, J. D., Twenter, B. M. & Gendrineau, T. Synthesis of substituted phenazines via palladium-catalyzed aryl ligation. Heterocycles 84, 1345–1353 (2012).
Fang, W., Liu, X., Lu, Z. & Tu, T. Photoresponsive metallo-hydrogels based on visual discrimination of the positional isomers through selective thixotropic gel collapse. Chem. Commun. 50, 3313–3316 (2014).
Frisch, M. J. et al. Gaussian 16 Revision C.01 (Gaussian Inc., 2016).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Clark, T., Chandrasekhar, J., Spitznagel, G. W. & Schleyer, P. V. R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 4, 294–301 (1983).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973).
Hehre, W. J., Ditchfield, R. & Pople, J. A. Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Acknowledgements
Yayuan Liu. and T.A.H. acknowledge support from the National Science Foundation (grant number 2029442). Yayuan Liu and X.L. acknowledge support from the Johns Hopkins University and American Chemical Society Petroleum Research Fund (65626-DNI4). Yuanyue Liu acknowledges support from the National Science Foundation (grant numbers 1900039 and 2029442), American Chemical Society Petroleum Research Fund (60934-DNI6) and Welch Foundation (grant number F-1959-20210327). For the calculations, we used computational resources at the Extreme Science and Engineering Discovery Environment, Texas Advanced Computing Center, Argonne National Laboratory and Brookhaven National Laboratory. We thank K. M. Diederichsen, H. Seo and E. Wenbo Zhao for helpful discussions.
Author information
Authors and Affiliations
Contributions
Yayuan Liu conceived of the project and designed the experiments. Yayuan Liu and X.L. carried out the experiments and analysed the data. X.Z. performed the DFT calculations. Yayuan Liu, Yuanyue Liu and T.A.H. supervised the project. Yayuan Liu, X.L. and X.Z. wrote the paper. All authors discussed the results and revised or commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Rajeev Assary and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Tables 1–9 and Notes 1–4.
Source data
Source Data Fig. 2
Cyclic voltammetry data for panels a–f.
Source Data Fig. 4
Cyclic voltammetry data for panels a–c and e.
Source Data Fig. 5
Time versus CO2 concentration data for each capture–release cycle.
Source Data Fig. 6
Time versus CO2 concentration data for each capture–release cycle and capture–release voltage profiles for panel d.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhao, X., Liu, Y. et al. Redox-tunable Lewis bases for electrochemical carbon dioxide capture. Nat Energy 7, 1065–1075 (2022). https://doi.org/10.1038/s41560-022-01137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01137-z
This article is cited by
-
Redox-tunable isoindigos for electrochemically mediated carbon capture
Nature Communications (2024)
-
Redox-mediated electrochemical liquid–liquid extraction for selective metal recovery
Nature Chemical Engineering (2024)
-
A phenazine-based high-capacity and high-stability electrochemical CO2 capture cell with coupled electricity storage
Nature Energy (2023)
-
Electrifying climate change mitigation
Nature Energy (2022)