Abstract
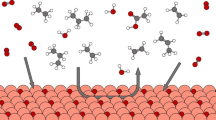
Direct non-oxidative dehydrogenation of alkanes produces useful carbon feedstocks and hydrogen fuel. However, breaking the C–H bonds in alkanes typically requires high temperature, stoichiometric oxidants or high-energy ultraviolet light; processes that operate under milder conditions are attractive but tend to have poor efficiency. Here we report Pt/black TiO2 photocatalysts in which Pt species are close to each other but not directly bonded, exhibiting high performance for alkane dehydrogenation in visible to near-infrared light at room temperature. For cyclohexane dehydrogenation, the turnover number for H2 production exceeded 100,000 without any deactivation over 80 reaction cycles, far beyond thermal reactions. For methane, 8.2% conversion was achieved with 65% selectivity to propane, rather than the more common ethane. We propose that methane undergoes intramolecular dehydrogenation to produce a methylene intermediate. For C2+ alkanes, fast dehydrogenation (up to 1,440 µmol g−1 h−1) to the corresponding olefins was realized. Distinct from isolated Pt+ monomers, the collections of Pt+ monomers give better photocatalytic activity and selectivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and Supplementary Information files. Atomic coordinates of the computational studies are provided as Supplementary Data 1. Source data are provided with this paper.
References
Bergman, R. G. C–H activation. Nature 446, 391–393 (2007).
Choudhary, V. R., Kinage, A. K. & Choudhary, T. V. Low-temperature nonoxidative activation of methane over H-galloaluminosilicate (MFI) zeolite. Science 275, 1286–1288 (1997).
Chen, H., Schlecht, S., Semple, T. C. & Hartwig, J. F. Thermal, catalytic, regiospecific functionalization of alkanes. Science 287, 1995–1997 (2000).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Yuliati, L. & Yoshida, H. Photocatalytic conversion of methane. Chem. Soc. Rev. 37, 1592–1602 (2008).
Schlapbach, L. & Züttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353–358 (2001).
Preuster, P., Papp, C. & Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 50, 74–85 (2017).
Choudhary, T. V., Aksoylu, E. & Wayne Goodman, D. Nonoxidative activation of methane. Catal. Rev. 45, 151–203 (2003).
Lytken, O., Lew, W. & Campbell, C. T. Catalytic reaction energetics by single crystal adsorption calorimetry: hydrocarbons on Pt(111). Chem. Soc. Rev. 37, 2172–2179 (2008).
Esswein, A. J. & Nocera, D. G. Hydrogen production by molecular photocatalysis. Chem. Rev. 107, 4022–4047 (2007).
Kato, Y., Yoshida, H. & Hattori, T. Photoinduced non-oxidative coupling of methane over silica-alumina and alumina around room temperature. Chem. Commun. 21, 2389–2390 (1998).
Yoshida, H., Matsushita, N., Kato, Y. & Hattori, T. Synergistic active sites on SiO2–Al2O3–TiO2 photocatalysts for direct methane coupling. J. Phys. Chem. B 107, 8355–8362 (2003).
Li, L. et al. Efficient sunlight-driven dehydrogenative coupling of methane to ethane over a Zn+-modified zeolite. Angew. Chem. Int. Ed. 50, 8299–8303 (2011).
Chowdhury, A. D. et al. Towards a practical development of light-driven acceptorless alkane dehydrogenation. Angew. Chem. Int. Ed. 53, 6477–6481 (2014).
Meng, L. et al. Gold plasmon-induced photocatalytic dehydrogenative coupling of methane to ethane on polar oxide surfaces. Energy Environ. Sci. 11, 294–298 (2018).
Wu, S. et al. Ga-doped and Pt-loaded porous TiO2–SiO2 for photocatalytic nonoxidative coupling of methane. J. Am. Chem. Soc. 141, 6592–6600 (2019).
Song, H., Meng, X., Wang, Z.-J., Liu, H. & Ye, J. Solar-energy-mediated methane conversion. Joule 3, 1606–1636 (2019).
Samantaray, M. K. et al. The comparison between single atom catalysis and surface organometallic catalysis. Chem. Rev. 120, 734–813 (2019).
Sun, G. et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 9, 4454 (2018).
Guo, X. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344, 616–619 (2014).
Naldoni, A. et al. Photocatalysis with reduced TiO2: from black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 9, 345–364 (2019).
Wang, Z. et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 23, 5444–5450 (2013).
Chen, Y. et al. Single-atom catalysts: synthetic strategies and electrochemical applications. Joule 2, 1242–1264 (2018).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Koel, B. E., Blank, D. A. & Carter, E. A. Thermochemistry of the selective dehydrogenation of cyclohexane to benzene on Pt surfaces. J. Mol. Catal. A 131, 39–53 (1998).
Li, L., Fan, S., Mu, X., Mi, Z. & Li, C.-J. Photoinduced conversion of methane into benzene over GaN nanowires. J. Am. Chem. Soc. 136, 7793–7796 (2014).
Kariya, N. et al. Efficient hydrogen production using cyclohexane and decalin by pulse-spray mode reactor with Pt catalysts. Appl. Catal. A 247, 247–259 (2003).
Lee, M.-H., Nagaraja, B. M., Lee, K. Y. & Jung, K.-D. Dehydrogenation of alkane to light olefin over PtSn/θ–Al2O3 catalyst: effects of Sn loading. Catal. Today 232, 53–62 (2014).
Marcinkowski, M. D. et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat. Chem. 10, 325–332 (2018).
Li, L., Mu, X.-Y., Liu, W., Mi, Z. & Li, C.-J. Simple and efficient system for combined solar energy harvesting and reversible hydrogen storage. J. Am. Chem. Soc. 137, 7576–7579 (2015).
Caballero, A. et al. Silver-catalyzed C–C bond formation between methane and ethyl diazoacetate in supercritical CO2. Science 332, 835–838 (2011).
Tan, H. et al. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 6, 10216–10223 (2014).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45, 13244–13249 (1992).
Yang, K., Dai, Y., Huang, B. & Feng, Y. P. Density-functional characterization of antiferromagnetism in oxygen-deficient anatase and rutile TiO2. Phys. Rev. B 81, 033202 (2010).
Arroyo-de Dompablo, M., Morales-Garcia, A. & Taravillo, M. DFT+U calculations of crystal lattice, electronic structure, and phase stability under pressure of TiO2 polymorphs. J. Chem. Phys. 135, 054503 (2011).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Hu, Z. & Metiu, H. Choice of U for DFT+U calculations for titanium oxides. J. Phys. Chem. C. 115, 5841–5845 (2011).
Gautam, G. S. & Carter, E. A. Evaluating transition metal oxides within DFT-SCAN and SCAN+U frameworks for solar thermochemical applications. Phys. Rev. Mater. 2, 095401 (2018).
Yu, X. et al. First principles calculations of electronic and optical properties of Mo-doped rutile TiO2. J. Alloys Compd 507, 33–37 (2010).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Ren, Y. et al. Unraveling the coordination structure–performance relationship in Pt1/Fe2O3 single-atom catalyst. Nat. Commun. 10, 4500 (2019).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant number 92061105, 21875090), Natural Science Foundation of Jilin Province (20210101121JC, 20210509035RQ) and the Fundamental Research Funds for the Central Universities. The XAFS experiments were conducted in 1W1B beam line of Beijing Synchrotron Radiation Facility. We thank Y. Zhao at Beijing University of Chemical Technology for help with XAFS experiments.
Author information
Authors and Affiliations
Contributions
L.Z. performed the catalyst design, synthesis, characterization, methane and cyclohexane conversion tests and data analysis. L. Liu. participated in the catalyst preparation and C2+ alkane tests. Z.G. and G.W. participated in the characterization. X.M. and K.H. provided helpful discussion. Z.P., R.Z., F.B. and Z.C. performed the DFT calculations. Y.W. and W.Z. performed the AC-HAADF-STEM measurements. L. Li. designed the study, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Jier Huang, Ding Ma, Hisao Yoshida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes, Figs. 1–44, Tables 1–8 and refs. 1–11.
Supplementary Data
Atomic coordinates for the Pt–Cl doping and Pt–O doping slabs.
Source data
Source Data Fig. 3
Data for Fig. 3.
Source Data Fig. 4
Data for Fig. 4.
Source Data Fig. 5
Data for Fig. 5.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Liu, L., Pan, Z. et al. Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions. Nat Energy 7, 1042–1051 (2022). https://doi.org/10.1038/s41560-022-01127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01127-1
This article is cited by
-
Incorporating metal nanoparticles in porous materials via selective heating effect using microwave
Nano Research (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
High-density frustrated Lewis pairs based on Lamellar Nb2O5 for photocatalytic non-oxidative methane coupling
Nature Communications (2023)
-
Photocatalytic nonoxidative coupling of methane to ethylene over carbon-doped ZnO/Au catalysts
Science China Chemistry (2023)