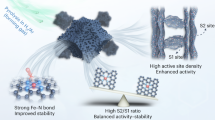
Abstract
Highly active and durable platinum group metal-free catalysts for the oxygen reduction reaction, such as Fe–N–C materials, are needed to lower the cost of proton-exchange membrane fuel cells. However, their durability is impaired by the attack of oxidizing radicals such as ·OH and HO2· that form from incomplete reduction of O2 via H2O2. Here we demonstrate that Ta–TiOx nanoparticle additives protect Fe–N–C catalysts from such degradation via radical scavenging. The 5 nm Ta–TiOx nanoparticles were uniformly synthesized on a Ketjenblack substrate using a high-temperature pulse technique, forming the rutile TaO2 phase. We found that Ta–TiOx nanoparticles suppressed the H2O2 yield by 51% at 0.7 V in an aqueous rotating ring disk electrode test. After an accelerated durability test, a fuel cell prepared with the scavengers showed a current density decay of 3% at 0.9 ViR-free (internal resistance-compensated voltage); a fuel cell without scavengers showed 33% decay. Thus, addition of Ta–TiOx provides an active defence strategy to improve the durability of oxygen reduction reaction catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files. Source data are provided with this paper.
References
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Wang, X. X., Swihart, M. T. & Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2, 578–589 (2019).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Jasinski, R. A new fuel cell cathode catalyst. Nature 201, 1963–1964 (1964).
Gewirth, A. A., Varnell, J. A. & Diascro, A. M. Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems. Chem. Rev. 118, 2313–2339 (2018).
Setzler, B. P., Zhuang, Z., Wittkopf, J. A. & Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 11, 1020–1025 (2016).
Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143–14149 (2017).
Primbs, M. et al. Establishing reactivity descriptors for platinum group metal (PGM)-free Fe–N–C catalysts for PEM fuel cells. Energy Environ. Sci. 13, 2480–2500 (2020).
Luo, F. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 19, 1215–1223 (2020).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J. P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Proietti, E. et al. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2, 416 (2011).
Zhang, G., Chenitz, R., Lefèvre, M., Sun, S. & Dodelet, J. P. Is iron involved in the lack of stability of Fe/N/C electrocatalysts used to reduce oxygen at the cathode of PEM fuel cells? Nano Energy 29, 111–125 (2016).
Shao, Y., Dodelet, J. P., Wu, G. & Zelenay, P. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Adv. Mater. 31, 1807615 (2019).
Choi, C. H. et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 11, 3176–3182 (2018).
Chenitz, R. et al. A specific demetalation of Fe–N4 catalytic sites in the micropores of NC–Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 11, 365–382 (2018).
Schmies, H. et al. Unravelling degradation pathways of oxide-supported Pt fuel cell nanocatalysts under in situ operating conditions. Adv. Energy Mater. 8, 1701663 (2018).
Xie, X. et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells. Nat. Catal. https://doi.org/10.1038/s41929-020-00546-1 (2020).
Jaouen, F. O2 reduction mechanism on non-noble metal catalysts for PEM fuel cells. Part II: a porous-electrode model to predict the quantity of H2O2 detected by rotating ring-disk electrode. J. Phys. Chem. C. 113, 15433–15443 (2009).
Lefèvre, M. & Dodelet, J. P. Fe-based catalysts for the reduction of oxygen in polymer electrolyte membrane fuel cell conditions: determination of the amount of peroxide released during electroreduction and its influence on the stability of the catalysts. Electrochim. Acta 48, 2749–2760 (2003).
Kumar, K. et al. On the influence of oxygen on the degradation of Fe–N–C catalysts. Angew. Chem. 132, 3261–3269 (2020).
Goellner, V., Armel, V., Zitolo, A., Fonda, E. & Jaouen, F. Degradation by hydrogen peroxide of metal–nitrogen–carbon catalysts for oxygen reduction. J. Electrochem. Soc. 162, H403–H414 (2015).
Qiao, Z. et al. 3D porous graphitic nanocarbon for enhancing the performance and durability of Pt catalysts: a balance between graphitization and hierarchical porosity. Energy Environ. Sci. 12, 2830–2841 (2019).
Gadipelli, S., Zhao, T., Shevlin, S. A. & Guo, Z. Switching effective oxygen reduction and evolution performance by controlled graphitization of a cobalt–nitrogen–carbon framework system. Energy Environ. Sci. 9, 1661–1667 (2016).
Gouérec, P. et al. Dioxygen reduction electrocatalysis in acidic media: effect of peripheral ligand substitution on cobalt tetraphenylporphyrin. J. Electroanal. Chem. 398, 67–75 (1995).
Gouérec, P. et al. Oxygen reduction in acid media catalysed by heat treated cobalt tetraazaannulene supported on an active charcoal: correlations between the performances after longevity tests and the active site configuration as seen by XPS and ToF-SIMS. J. Electroanal. Chem. 422, 61–75 (1997).
Bezerra, C. W. B. et al. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 173, 891–908 (2007).
Alves, M. C. M., Dodelet, J. P., Guay, D., Ladouceur, M. & Tourillon, G. Origin of the electrocatalytic properties for O2 reduction of some heat-treated polyacrylonitrile and phthalocyanine cobalt compounds adsorbed on carbon black as probed by electrochemistry and X-ray absorption spectroscopy. J. Phys. Chem. 96, 10898–10905 (1992).
Ladouceur, M. et al. Pyrolyzed cobalt phthalocyanine as electrocatalyst for oxygen reduction. J. Electrochem. Soc. 140, 1974–1981 (2019).
Kolthoff, H. M. & Medalia, A. I. The reaction between ferrous iron and peroxides. III. Reaction with cumene hydroperoxide, in aqueous solution. J. Am. Chem. Soc. 71, 3789–3792 (1949).
Kramm, U. I. et al. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 138, 635–640 (2016).
Jaouen, F. & Dodelet, J. P. O2 reduction mechanism on non-noble metal catalysts for PEM fuel cells. Part I: experimental rates of O2 electroreduction, H2O2 electroreduction, and H2O2 disproportionation. J. Phys. Chem. C. 113, 15422–15432 (2009).
Xie, H. et al. High-temperature pulse method for nanoparticle redispersion. J. Am. Chem. Soc. 142, 17364–17371 (2020).
Syono, Y., Kikuchi, M., Goto, T. & Fukuoka, K. Formation of rutile-type Ta(IV)O2 by shock reduction and cation-deficient Ta0.8O2 by subsequent oxidation. J. Solid State Chem. 50, 133–137 (1983).
Edgecomb, J. et al. Mapping localized peroxyl radical generation on a PEM fuel cell catalyst using integrated scanning electrochemical cell microspectroscopy. Front. Chem. 8, 572563 (2020).
Prabhakaran, V., Arges, C. G. & Ramani, V. Investigation of polymer electrolyte membrane chemical degradation and degradation mitigation using in situ fluorescence spectroscopy. Proc. Natl Acad. Sci. USA 109, 1029–1034 (2012).
Matuszak, Z., Reszka, K. J. & Chignell, C. F. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 23, 367–372 (1997).
Plauck, A., Stangland, E. E., Dumesic, J. A. & Mavrikakis, M. Active sites and mechanisms for H2O2 decomposition over Pd catalysts. Proc. Natl Acad. Sci. USA 113, E1973–E1982 (2016).
Laursen, A. B., Man, I. C., Trinhammer, O. L., Rossmeisl, J. & Dahl, S. The Sabatier principle illustrated by catalytic H2O2 decomposition on metal surfaces. J. Chem. Educ. 88, 1711–1715 (2011).
Balbuena, P. B., Calvo, S. R., Lamas, E. J., Salazar, P. F. & Seminario, J. M. Adsorption and dissociation of H2O2 on Pt and Pt-alloy clusters and surfaces. J. Phys. Chem. B 110, 17452–17459 (2006).
Poole, C. P. Electron Spin Resonance: A Comprehensive Treatise on Experimental Techniques 2nd edn, (Wiley, 1983).
Lee, Y.-K., Whittaker, M. M. & Whittaker, J. W. The electronic structure of the Cys-Tyrúÿfree radical in galactose oxidase determined by EPR spectroscopy. Biochemistry 47, 6637–6649 (2008).
Kresse, G. & Furthmüller, J. Bayesian optimization for calibrating and selecting hybrid-density functional models. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
L.H., Y.S., X.X. and H.X. acknowledge support from the US Department of Energy, Office of Energy Efficiency and Renewable Energy and Hydrogen and Fuel Cell Technologies Office through the Electrocatalysis consortium (ElectroCat) and from DOE programme managers D. Papageorgopoulos, S. Thompson, D. Peterson and G. Kleen. R.S.-Y. acknowledges financial support from National Science Foundation DMR-1809439. D.J. acknowledges support from the National Science Foundation CHE-2102191. G.H. acknowledges the financial support from the Queens College, City University of New York. The authors acknowledge the support of the Maryland Nanocenter, its Surface Analysis Center and AIMLab. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the US government or any agency thereof. Neither the US government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights.
Author information
Authors and Affiliations
Contributions
L.H. and Y.S. co-supervised the research. L.H., Y.S., V.P. and H.X. conceived the concept. H.X., X.X., Y.S. and L.H. designed the experiment. H.X. and X.X. carried out the experimental works including syntheses and electrochemical measurements. H.X., M.H., M.W. and A.H.P. carried out the characterization of materials. H.X., L.G.-L. and M.I.A.-S. carried out the EPR test. V.P. carried out the fluorescence test. S.S. and V.R. conducted the fuel cell test. G.H. and D.J. carried out the calculation analysis. H.X. and L.H. wrote the paper. G.H, X.X., R.S.-Y. and Y.S. revised the paper. All authors contributed to the discussion of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Frédéric Jaouen, Ulrike Kramm and Samira Siahrostami for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–22 and Table 1.
Supplementary Data
Source data for supplementary figures.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Rights and permissions
About this article
Cite this article
Xie, H., Xie, X., Hu, G. et al. Ta–TiOx nanoparticles as radical scavengers to improve the durability of Fe–N–C oxygen reduction catalysts. Nat Energy 7, 281–289 (2022). https://doi.org/10.1038/s41560-022-00988-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-00988-w
This article is cited by
-
Optimal geometrical configuration and oxidation state of cobalt cations in spinel oxides to promote the performance of Li-O2 battery
Nano Research (2024)
-
A stable atmospheric-pressure plasma for extreme-temperature synthesis
Nature (2023)
-
Unravelling the complex causality behind Fe–N–C degradation in fuel cells
Nature Catalysis (2023)
-
Improving bifunctional catalytic activity of biochar via in-situ growth of nickel-iron hydroxide as cathodic catalyst for zinc-air batteries
Biochar (2023)
-
Carbon-based supports for the electrocatalysis under industrially relevant conditions
Science China Chemistry (2023)