Abstract
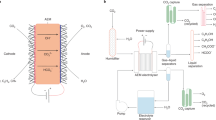
The alkaline environment of hydroxide exchange membrane fuel cells (HEMFCs) potentially allows use of cost-effective catalysts and bipolar plates in devices. However, HEMFC performance is adversely affected by CO2 present in the ambient air feed. Here, we demonstrate an electrochemically driven CO2 separator (EDCS) to remove CO2 from the air feed using a shorted membrane that conducts both anions and electrons. This EDCS is powered by hydrogen like a fuel cell but needs no electrical wires, bipolar plates or current collectors, and thus can be modularized like a typical separation membrane. We show that a 25 cm2 shorted membrane EDCS can achieve >99% CO2 removal from 2,000 standard cubic centimetres per minute (sccm) of air for 450 hours and operate effectively under load-following dynamic conditions. A spiral-wound EDCS module can remove >98% CO2 from 10,000 sccm of air. Our technoeconomic analysis indicates a compact and efficient module at >99% CO2 removal costs US$112 for an 80 kWnet HEMFC stack.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data presented in this study are available in figshare47 at https://doi.org/10.6084/m9.figshare.16744258.
References
US Department of Energy. Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan Section 3.4 https://www.energy.gov/eere/fuelcells/articles/hydrogen-and-fuel-cell-technologies-office-multi-year-research-development (2016).
Setzler, B. P., Zhuang, Z., Wittkopf, J. A. & Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 11, 1020–1025 (2016).
Gu, S., Xu, B. & Yan, Y. Electrochemical energy engineering: a new frontier of chemical engineering innovation. Ann. Rev. Chem. Biomol. Eng. 5, 429–454 (2014).
Campos-Roldán, C. A. & Alonso-Vante, N. The hydrogen oxidation reaction in alkaline medium: an overview. Electrochem. Energy Rev. 2, 312–331 (2019).
Davydova, E. S., Mukerjee, S., Jaouen, F. & Dekel, D. R. Electrocatalysts for hydrogen oxidation reaction in alkaline electrolytes. ACS Catal. 8, 6665–6690 (2018).
Ge, X. et al. Oxygen reduction in alkaline media: from mechanisms to recent advances of catalysts. ACS Catal. 5, 4643–4667 (2015).
Zhang, J. et al. Recent insights on catalyst layers for anion exchange membrane fuel cells. Adv. Sci. https://doi.org/10.1002/advs.202100284 (2021).
Huang, G. et al. Composite poly(norbornene) anion conducting membranes for achieving durability, water management and high power (3.4 W/cm2) in hydrogen/oxygen alkaline fuel cells. J. Electrochem. Soc. 166, F637–F644 (2019).
Wang, L., Peng, X., Mustain, W. E. & Varcoe, J. R. Radiation-grafted anion-exchange membranes: the switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 12, 1575–1579 (2019).
Wang, L., Bellini, M., Miller, H. A. & Varcoe, J. R. A high conductivity ultrathin anion-exchange membrane with 500+ h alkali stability for use in alkaline membrane fuel cells that can achieve 2 W cm−2 at 80 °C. J. Mater. Chem. A 6, 15404–15412 (2018).
Chen, N. et al. Poly(alkyl-terphenyl piperidinium) ionomers and membranes with an outstanding alkaline-membrane fuel-cell performance of 2.58 W cm(-2). Angew. Chem. Int. Ed. Engl. 60, 7710–7718 (2021).
Chen, N. et al. Poly(fluorenyl aryl piperidinium) membranes and ionomers for anion exchange membrane fuel cells. Nat. Commun. 12, 2367 (2021).
Fan, J. et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 10, 2306 (2019).
Lee, W.-H., Kim, Y. S. & Bae, C. Robust hydroxide ion conducting poly(biphenyl alkylene)s for alkaline fuel cell membranes. ACS Macro Lett. 4, 814–818 (2015).
Olsson, J. S., Pham, T. H. & Jannasch, P. Poly(arylene piperidinium) hydroxide ion exchange membranes: synthesis, alkaline stability, and conductivity. Adv. Funct. Mater. https://doi.org/10.1002/adfm.201702758 (2018).
Wang, Y. et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells. Nat. Commun. 10, 1506 (2019).
Adabi, H. et al. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 6, 834–843 (2021).
Peng, X. et al. Nitrogen-doped carbon–CoOx nanohybrids: a precious metal free cathode that exceeds 1.0 W cm−2 peak power and 100 h life in anion-exchange membrane fuel cells. Angew. Chem. Int. Ed. 58, 1046–1051 (2019).
Woo, J. et al. Promoting oxygen reduction reaction activity of Fe–N/C electrocatalysts by silica-coating-mediated synthesis for anion-exchange membrane fuel cells. Chem. Mater. 30, 6684–6701 (2018).
Lu, Y. et al. Halloysite-derived nitrogen doped carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 372, 82–90 (2017).
Xin, L., Zhang, Z., Wang, Z., Qi, J. & Li, W. Carbon supported Ag nanoparticles as high performance cathode catalyst for H2/O2 anion exchange membrane fuel cell. Front Chem. 1, 16 (2013).
Peng, X. et al. Using operando techniques to understand and design high performance and stable alkaline membrane fuel cells. Nat. Commun. 11, 3561 (2020).
Ul Hassan, N. et al. Achieving high‐performance and 2000 h stability in anion exchange membrane fuel cells by manipulating ionomer properties and electrode optimization. Adv. Energy Mater. https://doi.org/10.1002/aenm.202001986 (2020).
Inaba, M. et al. Effects of carbon dioxide on the performance of anion-exchange membrane fuel cells. Electrochemistry 79, 322–325 (2011).
Gerhardt, M. R., Pant, L. M. & Weber, A. Z. Along-the-channel impacts of water management and carbon-dioxide contamination in hydroxide-exchange-membrane fuel cells: a modeling study. J. Electrochem. Soc. 166, F3180–F3192 (2019).
Zheng, Y. et al. Quantifying and elucidating the effect of CO2 on the thermodynamics, kinetics and charge transport of AEMFCs. Energy Environ. Sci. 12, 2806–2819 (2019).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Caskey, S. R., Wong-Foy, A. G. & Matzger, A. J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 130, 10870–10871 (2008).
Shi, X. et al. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. Engl. 59, 6984–7006 (2020).
Yazaydın, A. Ö. et al. Screening of metal-organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J. Am. Chem. Soc. 131, 18198–18199 (2009).
Drese, J. H. et al. Synthesis–structure–property relationships for hyperbranched aminosilica CO2 adsorbents. Adv. Funct. Mater. 19, 3821–3832 (2009).
Wurzbacher, J. A., Gebald, C., Piatkowski, N. & Steinfeld, A. Concurrent separation of CO2 and H2O from air by a temperature-vacuum swing adsorption/desorption cycle. Environ. Sci. Technol. 46, 9191–9198 (2012).
Digdaya, I. A. et al. A direct coupled electrochemical system for capture and conversion of CO2 from oceanwater. Nat. Commun. 11, 4412 (2020).
Muroyama, A. P., Beard, A., Pribyl-Kranewitter, B. & Gubler, L. Separation of CO2 from dilute gas streams using a membrane electrochemical cell. ACS EST Eng. 1, 905–916 (2021).
Muroyama, A. P., Pătru, A. & Gubler, L. Review—CO2 separation and transport via electrochemical methods. J. Electrochem. Soc. https://doi.org/10.1149/1945-7111/abbbb9 (2020).
Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. ACS Catal. 10, 13058–13074 (2020).
Rigdon, W. A. et al. Carbonate dynamics and opportunities with low temperature, anion exchange membrane-based electrochemical carbon dioxide separators. J. Electrochem. Energy Convers. Storage https://doi.org/10.1115/1.4033411 (2017).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Shu, Q., Legrand, L., Kuntke, P., Tedesco, M. & Hamelers, H. V. M. Electrochemical regeneration of spent alkaline absorbent from direct air capture. Environ. Sci. Technol. 54, 8990–8998 (2020).
Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 12, 3530–3547 (2019).
Willauer, H. D., DiMascio, F., Hardy, D. R. & Williams, F. W. Feasibility of CO2 extraction from seawater and simultaneous hydrogen gas generation using a novel and robust electrolytic cation exchange module based on continuous electrodeionization technology. Ind. Eng. Chem. Res. 53, 12192–12200 (2014).
Willauer, H. D., DiMascio, F., Hardy, D. R. & Williams, F. W. Development of an electrolytic cation exchange module for the simultaneous extraction of carbon dioxide and hydrogen gas from natural seawater. Energy Fuels 31, 1723–1730 (2017).
Matz, S. et al. Demonstration of electrochemically-driven CO2 separation using hydroxide exchange membranes. J. Electrochem. Soc. https://doi.org/10.1149/1945-7111/abd5fe (2021).
Zheng, Y. et al. Editors’ choice—power-generating electrochemical CO2 scrubbing from air enabling practical AEMFC application. J. Electrochem. Soc. https://doi.org/10.1149/1945-7111/abe08a (2021).
Baker, D. R., Caulk, D. A., Neyerlin, K. C. & Murphy, M. W. Measurement of oxygen transport resistance in PEM fuel cells by limiting current methods. J. Electrochem. Soc. 156, B991 (2009).
Shi, L. et al. Editors’ choice—uncovering the role of alkaline pretreatment for hydroxide exchange membrane fuel cells. J. Electrochem. Soc. https://doi.org/10.1149/1945-7111/abc4bd (2020).
Shi, L. et al. A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells. figshare https://doi.org/10.6084/m9.figshare.16744258 (2022).
US Department of Energy Office of Energy Efficiency and Renewable Energy. Fuel Economy www.fueleconomy.gov (2021).
Acknowledgements
The information, data or work presented herein were funded in part by the Advanced Research Projects Agency-Energy (ARPA-E), US Department of Energy, under award number DE-AR0001034. S.G. is the principal investigator of the project. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Contributions
B.P.S. and Y.Y. conceived the idea of the shorted membrane and spiral-wound module. L.S. prepared the shorted membranes and designed the experiments. S.M. and B.P.S. designed the spiral-wound module. L.S. and Y.Z. characterized the shorted membranes, and fabricated and performed the single cell EDCS and spiral-wound module tests. B.P.S. developed the model of CO2 effect. B.P.S. and Y.Z. completed the technoeconomic analysis. S.G. provided guidance throughout the project, helped with data interpretation and improved the manuscript. L.S., Y.Z., B.P.S. and Y.Y. wrote the manuscript with the support of all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The PiperION membranes and ionomers were provided for free by Versogen, Inc. for which Y.Y., B.P.S. and Y.Z. are cofounders and S.G. is an adviser. L.S. and S.M. claim no competing interests.
Peer review
Peer review information
Nature Energy thanks Lorenz Gubler, Hamish Miller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Tables 1–4 and Figs. 1–16.
Supplementary Video 1
Video showing demonstration of the spiral-wound EDCS module with different rates of hydrogen supply.
Rights and permissions
About this article
Cite this article
Shi, L., Zhao, Y., Matz, S. et al. A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells. Nat Energy 7, 238–247 (2022). https://doi.org/10.1038/s41560-021-00969-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00969-5
This article is cited by
-
A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis
Nature Catalysis (2024)
-
Towards reliable assessment of hydrogen oxidation electrocatalysts for anion-exchange membrane fuel cells
Nano Research (2023)
-
Wire-free electrochemical CO2 scrubbing
Nature Energy (2022)