Abstract
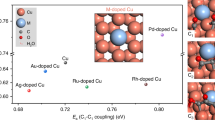
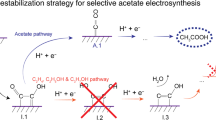
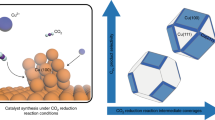
The high-energy-density C3 fuel n-propanol is desired from CO2/CO electroreduction, as evidenced by propanol’s high market price per tonne (approximately US$ 1,400–1,600). However, CO electroreduction to n-propanol has shown low selectivity, limited production rates and poor stability. Here we report catalysts, identified using computational screening, that simultaneously facilitate multiple carbon–carbon coupling, stabilize C2 intermediates and promote CO adsorption, all leading to improved n-propanol electrosynthesis. Experimentally we construct the predicted optimal electrocatalyst based on silver–ruthenium co-doped copper. We achieve, at 300 mA cm−2, a high n-propanol Faradaic efficiency of 36% ± 3%, a C2+ Faradaic efficiency of 93% and single-pass CO conversion of 85%. The system exhibits 100 h stable n-propanol electrosynthesis. Technoeconomic analysis based on the performance of the pilot system projects profitability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available within the paper, Supplementary Information and source data files. Source data are provided with this paper.
References
Jhong, H.-R., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191–199 (2013).
Wu, Y., Jiang, Z., Lu, X., Liang, Y. & Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Service, R. F. Hunt for renewable plastics clears a hurdle. Science 371, 873 (2021).
Hauch, A. et al. Recent advances in solid oxide cell technology for electrolysis. Science 370, eaba6118 (2020).
Küngas, R. Review—electrochemical CO2 reduction for CO production: comparison of low- and high-temperature electrolysis technologies. J. Electrochem. Soc. 167, 044508 (2020).
Skafte, T. L. et al. Selective high-temperature CO2 electrolysis enabled by oxidized carbon intermediates. Nat. Energy 4, 846–855 (2019).
Klabunde, J., Bischoff, C. & Papa, A. J. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, 2018).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 4614 (2018).
Zhuang, T.-T. et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal 1, 946–951 (2018).
Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Nat. Catal. 2, 251–258 (2019).
de Arquer, F. P. G. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Zhuang, T.-T. et al. Steering post-C-C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Xiao, H., Cheng, T. & Goddard, W. A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu (111). J. Am. Chem. Soc. 139, 130–136 (2016).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Wang, X. et al. Efficient upgrading of CO to C3 fuel using asymmetric C–C coupling active sites. Nat. Commun. 10, 5186 (2019).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Xiao, H., Goddard, W. A., Cheng, T. & Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl Acad. Sci. USA 114, 6685–6688 (2017).
Lum, Y., Cheng, T., Goddard, W. A. & Ager, J. W. Electrochemical CO reduction builds solvent water into oxygenate products. J. Am. Chem. Soc. 140, 9337–9340 (2018).
Hussain, J., Jonsson, H. & Skulason, E. Calculations of product selectivity in electrochemical CO2 reduction. ACS Catal. 8, 5240–5249 (2018).
Goodpaster, J. D., Bell, A. T. & Head-Gordon, M. Identification of possible pathways for C–C bond formation during electrochemical reduction of CO2: new theoretical insights from an improved electrochemical model. J. Phys. Chem. Lett. 7, 1471–1477 (2016).
Cobley, C. M. & Xia, Y. Engineering the properties of metal nanostructures via galvanic replacement reactions. Mater. Sci. Eng. R 70, 44–62 (2010).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Han, L., Wang, P., Liu, H., Tan, Q. & Yang, J. Balancing the galvanic replacement and reduction kinetics for the general formation of bimetallic CuM (M = Ru, Rh, Pd, Os, Ir, and Pt) hollow nanostructures. J. Mater. Chem. A 4, 18354–18365 (2016).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Zhou, Y. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 19, 974–980 (2018).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Sheppard, N. & Nguyen, T. T. in Advances in Infrared and Raman Spectroscopy (eds Clark, R. J. H. & Hester, R. E.) Ch. 5 (Heyden, 1978).
Gunathunge, C. M., Ovalle, V. J., Li, Y., Janik, M. J. & Waegele, M. M. Existence of an electrochemically inert CO population on Cu electrodes in alkaline pH. ACS Catal. 8, 7507–7516 (2018).
Fielicke, A., Gruene, P., Meijer, G. & Rayner, D. M. The adsorption of CO on transition metal clusters: a case study of cluster surface chemistry. Surf. Sci. 603, 1427–1433 (2009).
Sandberg, R. B., Montoya, J. H., Chan, K. & Nørskov, J. K. CO–CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Luc, W., Rosen, J. & Jiao, F. An Ir-based anode for a practical CO2 electrolyzer. Catal. Today 288, 79–84 (2017).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Rumble, J. R. CRC Handbook of Chemistry and Physics 99th edn (CRC Press, 2018).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada (number RGPIN-2017-06477, E.H.S.) and the Ontario Research Fund—Research Excellence Program (number ORF-RE08-034, E.H.S.). DFT calculations were performed on the Niagara supercomputer at the SciNet HPC Consortium. We acknowledge the computational resources supported by SciNet, which is funded by the University of Toronto, the Ontario Research Fund—Research Excellence Program, the Government of Ontario and the Canada Foundation for Innovation. D.S. acknowledges the NSERC E.W.R. Steacie Memorial Fellowship. Synchrotron measurements were carried out at the BL-17C at the National Synchrotron Radiation Research Center. We thank R. Wolowiec and D. Kopilovic for their kind technical assistance, Ontario Centre for the Characterization of Advanced Materials (OCCAM) of the University of Toronto and the National Synchrotron Radiation Research Center.
Author information
Authors and Affiliations
Contributions
E.H.S. supervised the project. X.W. and E.H.S. conceived the idea. X.W. designed and carried out the experiments. P.O. carried out DFT calculations. S.-F.H. performed XAS measurements. S.-F.H. and J.A. analysed the XAS data. A.O. fabricated the IrOx-coated Ti mesh electrodes. J.T. and J.Y.H. contributed to the SEM and TEM characterization. X.W and J.S. did the TEA. K.B. and ASR carried out XPS measurements. X.W. and M.S. performed XRD measurements. C.M.G. and F.P.G.d.A. contributed to the manuscript editing. X.W., P.O., and E.H.S. co-wrote the manuscript. R.K.M., C.P.O, Z.W., A.H.I. and D.S. assisted with the discussions. All authors discussed the results and assisted during manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
X.W. and E.H.S. have filed a provisional patent application titled ‘Manufacturing and use of co-doped multi-metallic electrocatalysts for upgrading of CO to propanol’ (application number 63/192,842). All other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Maximilian Fleischer, Xiaowa Nie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36, Tables 1–7, Note 1 and refs. 1–8.
Source data
Source Data Fig. 3
Source data for Fig. 3a–c.
Source Data Fig. 4
Source data for Fig. 4b.
Rights and permissions
About this article
Cite this article
Wang, X., Ou, P., Ozden, A. et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Nat Energy 7, 170–176 (2022). https://doi.org/10.1038/s41560-021-00967-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00967-7
This article is cited by
-
Dinuclear Cu(I) molecular electrocatalyst for CO2-to-C3 product conversion
Nature Catalysis (2024)
-
Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity
Nature Communications (2024)
-
Tandem reactors and reactions for CO2 conversion
Nature Chemical Engineering (2024)
-
Advances in bio-inspired electrocatalysts for clean energy future
Nano Research (2024)
-
Unlocking direct CO2 electrolysis to C3 products via electrolyte supersaturation
Nature Catalysis (2023)