Abstract
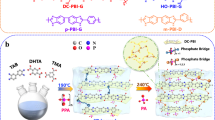
Conventional proton exchange membrane fuel cells (PEMFCs) operate within narrow temperature ranges. Typically, they are run at either 80‒90 °C using fully humidified perfluorosulfonic acid membranes, or at 140‒180 °C using non-humidified phosphoric acid (PA)-doped membranes, to avoid water condensation-induced PA leaching. However, the ability to function over a broader range of temperature and humidity could simplify heat and water management, thus reducing costs. Here we present PA-doped intrinsically ultramicroporous membranes constructed from rigid, high free volume, Tröger’s base-derived polymers, which allow operation from −20 to 200 °C. Membranes with an average ultramicropore radius of 3.3 Å show a syphoning effect that allows high retention of PA even under highly humidified conditions and present more than three orders of magnitude higher proton conductivity retention than conventional dense PA-doped polybenzimidazole membranes. The resulting PA-doped PEMFCs display 95% peak power density retention after 150 start-up/shut-down cycles at 15 °C and can accomplish over 100 cycles, even at −20 °C.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. The authors declare that the data supporting the findings of this study are available within the paper and Supplementary information. Further data beyond the immediate results presented here are available from the corresponding authors on reasonable.
Code availability
This study did not generate any datasets.
References
Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 1, 101–119 (2008).
Çelik, S. Ü. et al. Alternatives toward proton conductive anhydrous membranes for fuel cells: heterocyclic protogenic solvents comprising polymer electrolytes. Prog. Polym. Sci. 37, 1265–1291 (2012).
Mauritz, K. A. et al. State of understanding of Nafion. Chem. Rev. 104, 4535–4585 (2004).
Zhang, J. et al. High temperature PEM fuel cells. J. Power Sources 160, 872–891 (2006).
Zhu, Y. et al. Performance comparison between high temperature and traditional proton exchange membrane fuel cell stacks using electrochemical impedance spectroscopy. J. Power Sources 256, 250–257 (2014).
Schmidt, T. J. et al. Durability and reliability in high-temperature reformed hydrogen PEFCs. ECS Trans. 3, 861–869 (2006).
Bose, S. et al. Polymer membranes for high temperature proton exchange membrane fuel cell: recent advances and challenges. Prog. Polym. Sci. 36, 813–843 (2011).
Li, Q. et al. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 34, 449–477 (2009).
Ma, Y. L. et al. Conductivity of PBI membranes for high-temperature polymer electrolyte fuel cells. J. Electrochem. Soc. 151, A8–A16 (2004).
Yu, S. et al. Durability studies of PBI-based high temperature PEMFCs. Fuel Cells 8, 165–174 (2008).
Li, Q. et al. PBI-based polymer membranes for high temperature fuel cells—preparation, characterization and fuel cell demonstration. Fuel Cells 4, 147–158 (2004).
Xiao, L. et al. High-temperature polybenzimidazole fuel cell membranes via a sol-gel process. Chem. Mater. 17, 5328–5333 (2005).
Kim, J. et al. Durability of high temperature polymer electrolyte membrane fuel cells in daily based start/stop operation mode using reformed gas. Int. J. Hydrog. Energy 40, 7769–7776 (2015).
Lang, S. et al. Experimental investigation and numerical simulation of the electrolyte loss in a HT-PEM fuel cell. Int. J. Hydrog. Energy 40, 1163–1172 (2015).
Wei, L. et al. Numerical study of cold start performance of proton exchange membrane fuel cell with coolant circulation. Int. J. Hydrog. Energy 44, 22160–22172 (2019).
Oszcipok, M. et al. Portable proton exchange membrane fuel-cell systems for outdoor applications. J. Power Sources 157, 666–673 (2006).
Amamou, A. A. et al. Comprehensive review of solutions and strategies for cold start of automotive proton exchange membrane fuel cells. IEEE Access 4, 4989–5002 (2016).
Maruo, T. et al. Development of fuel cell system control for sub-zero ambient conditions. SAE Technical Paper Series, https://doi.org/10.4271/2017-01-1189 (2017).
Jiang, W. et al. Study on fast cold start-up method of proton exchange membrane fuel cell based on electric heating technology. Energies 13, 4456–4471 (2020).
Wen, J. et al. Alternate hydrogen pump method enables start-up from −30 °C for graphite-bipolar-plate proton exchange membrane fuel cells. J. Electrochem. Soc. 166, F1112–F1116 (2019).
Lee, K.-S. et al. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs. Nat. Energy 1, 16120–16126 (2016).
Lee, K.-S. et al. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. Energy Environ. Sci. 11, 979–987 (2018).
Lee, A. S. et al. The energetics of phosphoric acid interactions reveals a new acid loss mechanism. J. Mater. Chem. A 7, 9867–9876 (2019).
Atanasov, V. et al. Synergistically integrated phosphonated poly(pentafluorostyrene) for fuel cells. Nat. Mater. 20, 370–377 (2021).
McKeown, N. B. et al. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 35, 675–683 (2006).
Carta, M. et al. An efficient polymer molecular sieve for membrane gas separations. Science 339, 303–307 (2013).
Zuo, P. et al. Sulfonated microporous polymer membranes with fast and selective ion transport for electrochemical energy conversion and storage. Angew. Chem. Int. Ed. Engl. 59, 9564–9573 (2020).
Yang, Z. et al. Highly conductive anion-exchange membranes from microporous Tröger’s base polymers. Angew. Chem. Int. Ed. Engl. 55, 11499–11502 (2016).
Tan, R. et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage. Nat. Mater. 19, 195–202 (2020).
Patel, H. A. et al. Proton conduction in Tröger’s base-linked poly(crown ether)s. ACS Appl. Mater. Interfaces 10, 25303–25310 (2018).
Liu, X. et al. Oriented proton-conductive nano-sponge-facilitated polymer electrolyte membranes. Energy Environ. Sci. 13, 297–309 (2020).
Zhou, S. et al. Alkaline polymers of intrinsic microporosity: high-conduction and low-loss anhydrous proton exchange membranes for energy conversion. J. Mater. Chem. A 9, 3925–3930 (2021).
Xu, Z. et al. Preparation and antifouling property improvement of Tröger’s base polymer ultrafiltration membrane. J. Membr. Sci. 561, 59–68 (2018).
Carta, M. et al. The synthesis of microporous polymers using Tröger’s base formation. Polym. Chem. 5, 5267–5272 (2014).
Liao, K.-S. et al. Determination of free-volume properties in polymers without orthopositronium components in positron annihilation lifetime spectroscopy. Macromolecules 44, 6818–6826 (2011).
Provencher, S. W. Contin: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 27, 229–242 (1982).
Kirkegaard, P. et al. Program system for analysing positron lifetime spectra and angular correlation curves. Comput. Phys. Commun. 23, 307–335 (1981).
Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 82, 1059–1073 (2010).
Sebastiani, D. Current densities and nucleus-independent chemical shift maps from reciprocal-space density functional perturbation theory calculations. ChemPhysChem 7, 164–175 (2006).
Aihara, Y. et al. Ion conduction mechanisms and thermal properties of hydrated and anhydrous phosphoric acids studied with 1H, 2H, and 31P NMR. J. Phys. Chem. B 110, 24999–25006 (2006).
Xu, Z. et al. 31P NMR study on some phosphorus-containing compounds. Chin. Chem. Lett. 11, 1057–1060 (2000).
Harris, R. K. et al. Adsorption competition onto activated carbon, studied by magic-angle spinning NMR. J. Chem. Soc., Faraday Trans. 92, 2615–2618 (1996).
Dickinson, L. M. et al. Oxygen-17 and deuterium NMR investigation into the adsorption of water on activated carbon. Magn. Reson. Chem. 38, 918–924 (2000).
Zhang, E. et al. NMR analysis of phosphoric acid distribution in porous fuel cell catalysts. Chem. Commun. 57, 2547–2550 (2021).
Chesnut, D. B. A theoretical study of 31P NMR chemical shielding models for concentrated phosphoric acid solution. J. Phys. Chem. A 109, 11962–11966 (2005).
Milic, M. et al. NMR quantification of hydrogen-bond-accepting ability for organic molecules. J. Org. Chem. 86, 6031–6043 (2021).
Uosaki, K. et al. Conductivity of Nafion membranes at low temperatures. J. Electroanal. Chem. Interfacial Electrochem. 287, 163–169 (1990).
Soboleva, T. et al. Investigation of the through-plane impedance technique for evaluation of anisotropy of proton conducting polymer membranes. J. Electroanal. Chem. 622, 145–152 (2008).
Aili, D. et al. Thermal curing of PBI membranes for high temperature PEM fuel cells. J. Mater. Chem. 22, 5444–5453 (2012).
Aili, D. et al. Polybenzimidazole‑based high‑temperature polymer electrolyte membrane fuel cells: new insights and recent progress. Electrochem. Energy Rev. 3, 793–845 (2020).
Haider, R. et al. High temperature proton exchange membrane fuel cells: progress in advanced materials and key technologies. Chem. Soc. Rev. 50, 1138–1187 (2021).
Ogihara, W. et al. Proton-conducting ionic liquids based upon multivalent anions and alkylimidazolium cations. Chem. Commun. 3637–3639 (2006).
Fernicola, A. et al. New types of Brönsted acid–base ionic liquids-based membranes for applications in PEMFCs. ChemPhysChem 8, 1103–1107 (2007).
Yang, J. S. et al. High molecular weight polybenzimidazole membranes for high temperature PEMFC. Fuel Cells 14, 7–15 (2014).
Tang, H. Y. et al. Synthesis and properties of phosphonated polysulfones for durable high-temperature proton exchange membranes fuel cell. J. Membr. Sci. 605, 118107 (2020).
Marenich, A. V. et al. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Legault, C. Y. CYLview http://www.cylview.org/ (Univ. de Sherbrooke, 2020).
Gaussian 09 Rev. B.01 (Gaussian, 2009).
Acknowledgements
We thank T. Yan and Z. He from Nankai University for the computational study the alkalinity of N atoms. We also thank Y. Li from Soochow University (computational chemistry materials simulation and design) for the molecular dynamics simulation and X. Ma from Tiangong University for the synthesis of Trip-TB polymer. We are grateful to the National Natural Science Foundation of China (grant nos. 21835005 and 52G15023), Science and Technology Major Projects of Shanxi Province of China (grant no. 20181102019), the Hundred Talents Program of the Shanxi Province and the autonomous research project of SKLCC (grant no. 2020BWZ001).
Author information
Authors and Affiliations
Contributions
H.T. and K.G. designed the experiments, performed the polymer characterization, MEA fabrication, testing experiments of fuel cells and data collection. F.Y. helped with data collection. L.W. aided in the synthesis of TB polymers. J.L. and Z.C. measured the PALS of the membranes. W.Y. and M.D.G. supervised and guided the work. N.L. developed the concept and supervised the research. H.T., W.Y., N.L. and M.D.G. analysed all experimental data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Dirk Henkensmeier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Tables 1–4.
Supplementary Data 1
Calculated Cartesian coordinates for TB polymers.
Supplementary Data 2
Source Data for Supplementary Figs. 7 and 8 and Table 3.
Source data
Source Data Fig. 1
Chemical structures and properties of TB polymers.
Source Data Fig. 2
Solid-state 31P NMR spectra of PA-saturated samples.
Source Data Fig. 3
Conductivity and PA loss for PA-doped membranes under different conditions.
Source Data Fig. 4
i‒V curves, power density and HFR of MEAs without backpressure or external humidification.
Source Data Fig. 5
Figure 5: durability of cell performance.
Rights and permissions
About this article
Cite this article
Tang, H., Geng, K., Wu, L. et al. Fuel cells with an operational range of –20 °C to 200 °C enabled by phosphoric acid-doped intrinsically ultramicroporous membranes. Nat Energy 7, 153–162 (2022). https://doi.org/10.1038/s41560-021-00956-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00956-w
This article is cited by
-
An operationally broadened alkaline water electrolyser enabled by highly stable poly(oxindole biphenylene) ion-solvating membranes
Nature Energy (2024)
-
Solution-processable polymers of intrinsic microporosity for gas-phase carbon dioxide photoreduction
Nature Communications (2023)
-
Upscaled production of an ultramicroporous anion-exchange membrane enables long-term operation in electrochemical energy devices
Nature Communications (2023)
-
Near-frictionless ion transport within triazine framework membranes
Nature (2023)
-
Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly
Electrochemical Energy Reviews (2023)