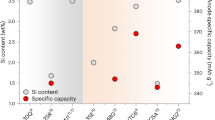
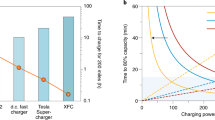
Abstract
High-energy batteries for automotive applications require cells to endure well over a decade of constant use, making their long-term stability paramount. This is particularly challenging for emerging cell chemistries containing silicon, for which extended testing information is scarce. While much of the research on silicon anodes has focused on mitigating the consequences of volume changes during cycling, comparatively little is known about the time-dependent degradation of silicon-containing batteries. Here we discuss a series of studies on the reactivity of silicon that, collectively, paint a picture of how the chemistry of silicon exacerbates the calendar aging of lithium-ion cells. Assessing and mitigating this shortcoming should be the focus of future research to fully realize the benefits of this battery technology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nykvist, B. & Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change 5, 329–332 (2015).
He, G. N., Ciez, R., Moutis, P., Kar, S. & Whitacre, J. F. The economic end of life of electrochemical energy storage. Appl. Energy 273, 115151 (2020).
Dubarry, M., Qin, N. & Brooker, P. Calendar aging of commercial Li-ion cells of different chemistries—a review. Curr. Opin. Electrochem. 9, 106–113 (2018).
Harlow, J. E. et al. A wide range of testing results on an excellent lithium-ion cell chemistry to be used as benchmarks for new battery technologies. J. Electrochem. Soc. 166, A3031–A3044 (2019).
Keil, P. et al. Calendar aging of lithium-ion batteries I. Impact of the graphite anode on capacity fade. J. Electrochem. Soc. 163, A1872–A1880 (2016).
Single, F., Latz, A. & Horstmann, B. Identifying the mechanism of continued growth of the solid–electrolyte interphase. ChemSusChem 11, 1950–1955 (2018).
Zilberman, I., Sturm, J. & Jossen, A. Reversible self-discharge and calendar aging of 18650 nickel-rich, silicon-graphite lithium-ion cells. J. Power Sources 425, 217–226 (2019).
Rodrigues, M. T. F., Kalaga, K., Trask, S. E., Shkrob, I. A. & Abraham, D. P. Anode-dependent impedance rise in layered-oxide cathodes of lithium-ion cells. J. Electrochem. Soc. 165, A1697–A1705 (2018).
Deng, Z. et al. Ultrasonic scanning to observe wetting and “unwetting” in Li-ion pouch cells. Joule 4, 2017–2029 (2020).
Naumann, M., Schimpe, M., Keil, P., Hesse, H. C. & Jossen, A. Analysis and modeling of calendar aging of a commercial LiFePO4/graphite cell. J. Energy Storage 17, 153–169 (2018).
Ding, Y. L., Cano, Z. P., Yu, A. P., Lu, J. & Chen, Z. W. Automotive Li-ion batteries: current status and future perspectives. Electrochem. Energy Rev. 2, 1–28 (2019).
Cunningham, B. Silicon and Intermetallic Anode Portfolio Strategy Overview Annual Merit Review (US Department of Energy, 2020).
Zilberman, I., Ludwig, S. & Jossen, A. Cell-to-cell variation of calendar aging and reversible self-discharge in 18650 nickel-rich, silicon–graphite lithium-ion cells. J. Energy Storage 26, 100900 (2019).
De Sutter, L. et al. Comprehensive aging analysis of volumetric constrained lithium-ion pouch cells with high concentration silicon-alloy anodes. Energies 11, 2948 (2018).
Lu, W., Zhang, L., Qin, Y. & Jansen, A. Calendar and cycle life of lithium-ion batteries containing silicon monoxide anode. J. Electrochem. Soc. 165, A2179–A2183 (2018).
Kalaga, K., Rodrigues, M. T. F., Trask, S. E., Shkrob, I. A. & Abraham, D. P. Calendar-life versus cycle-life aging of lithium-ion cells with silicon-graphite composite electrodes. Electrochim. Acta 280, 221–228 (2018).
Schneier, D. et al. Elucidation of the spontaneous passivation of silicon anodes in lithium battery electrolytes. J. Electrochem. Soc. 166, A4020–A4024 (2019).
Yin, Y. L. et al. Nonpassivated silicon anode surface. ACS Appl. Mater. Interfaces 12, 26593–26600 (2020).
Pekarek, R. T. et al. Intrinsic chemical reactivity of solid–electrolyte interphase components in silicon-lithium alloy anode batteries probed by FTIR spectroscopy. J. Mater. Chem. A 8, 7897–7906 (2020).
Seitzinger, C. L. et al. Intrinsic chemical reactivity of silicon electrode materials: gas evolution. Chem. Mater. 32, 3199–3210 (2020).
Aurbach, D. et al. On the use of vinylene carbonate (VC) electrolyte solutions for Li-ion as an additive to batteries. Electrochim. Acta 47, 1423–1439 (2002).
Yoon, I., Abraham, D. P., Lucht, B. L., Bower, A. F. & Guduru, P. R. In situ measurement of solid electrolyte interphase evolution on silicon anodes using atomic force microscopy. Adv. Energy Mater. 6, 1600099 (2016).
Veith, G. M. et al. Direct determination of solid–electrolyte interphase thickness and composition as a function of state of charge on a silicon anode. J. Phys. Chem. C 119, 20339–20349 (2015).
Hasa, I. et al. Electrochemical reactivity and passivation of silicon thin-film electrodes in organic carbonate electrolytes. ACS Appl. Mater. Interfaces 12, 40879–40890 (2020).
Shkrob, I. A., Wishart, J. F. & Abraham, D. P. What makes fluoroethylene carbonate different? J. Phys. Chem. C 119, 14954–14964 (2015).
Veith, G. M. et al. Determination of the solid electrolyte interphase structure grown on a silicon electrode using a fluoroethylene carbonate additive. Sci. Rep. 7, 6326 (2017).
Jung, R. et al. Consumption of fluoroethylene carbonate (FEC) on Si-C composite electrodes for Li-ion batteries. J. Electrochem. Soc. 163, A1705–A1716 (2016).
Bryngelsson, H., Stjerndahl, M., Gustafsson, T. & Edström, K. How dynamic is the SEI? J. Power Sources 174, 970–975 (2007).
Stetson, C. et al. Temperature-dependent solubility of solid electrolyte interphase on silicon electrodes. ACS Energy Lett. 4, 2770–2775 (2019).
Hou, T. Z. et al. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation. Nano Energy 64, 103881 (2019).
Gao, H. et al. Parasitic reactions in nanosized silicon anodes for lithium-ion batteries. Nano Lett. 17, 1512–1519 (2017).
Tornheim, A., Trask, S. E. & Zhang, Z. C. Evaluation of electrolyte oxidation stability on charged LiNi0.5Co0.2Mn0.3O2 cathode surface through potentiostatic holds. J. Electrochem. Soc. 163, A1717–A1722 (2016).
Barre, A. et al. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 241, 680–689 (2013).
Xu, H. et al. Roll-to-roll prelithiation of Sn foil anode suppresses gassing and enables stable full-cell cycling of lithium ion batteries. Energ. Environ. Sci. 12, 2991–3000 (2019).
Stich, M., Gottlinger, M., Kurniawan, M., Schmidt, U. & Bund, A. Hydrolysis of LiPF6 in carbonate-based electrolytes for lithium-ion batteries and in aqueous media. J. Phys. Chem. C 122, 8836–8842 (2018).
Wiemers-Meyer, S., Jeremias, S., Winter, M. & Nowak, S. Influence of battery cell components and water on the thermal and chemical stability of LiPF6 based lithium ion battery electrolytes. Electrochim. Acta 222, 1267–1271 (2016).
Burns, J. C. et al. The impact of intentionally added water to the electrolyte of Li-ion cells. J. Electrochem. Soc. 160, A2281–A2287 (2013).
Ha, Y. Y. et al. Effect of water concentration in LiPF6-based electrolytes on the formation, evolution, and properties of the solid electrolyte interphase on Si anodes. ACS Appl. Mater. Interfaces 12, 49563–49573 (2020).
Jung, C. H., Kim, K. H. & Hong, S. H. Stable silicon anode for lithium-ion batteries through covalent bond formation with a binder via esterification. ACS Appl. Mater. Interfaces 11, 26753–26763 (2019).
Bareno, J., Shkrob, I. A., Gilbert, J. A., Klett, M. & Abraham, D. P. Capacity fade and its mitigation in Li-ion cells with silicon-graphite electrodes. J. Phys. Chem. C 121, 20640–20649 (2017).
Shin, J., Kim, T. H., Lee, Y. & Cho, E. Key functional groups defining the formation of Si anode solid–electrolyte interphase towards high energy density Li-ion batteries. Energy Storage Mater. 25, 764–781 (2020).
Han, B. H. et al. Using mixed salt electrolytes to stabilize silicon anodes for lithium-ion batteries via in situ formation of Li–M–Si ternaries (M = Mg, Zn, Al, Ca). ACS Appl. Mater. Interfaces 11, 29780–29790 (2019).
Liu, N. et al. A yolk–shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 12, 3315–3321 (2012).
Hy, S. et al. Stabilizing nanosized Si anodes with the synergetic usage of atomic layer deposition and electrolyte additives for Li-ion batteries. ACS Appl. Mater. Interfaces 7, 13801–13807 (2015).
Wetjen, M. et al. Morphological changes of silicon nanoparticles and the influence of cutoff potentials in silicon-graphite electrodes. J. Electrochem. Soc. 165, A1503–A1514 (2018).
Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Han, J. G., Kim, K., Lee, Y. & Choi, N. S. Scavenging materials to stabilize LiPF6-containing carbonate-based electrolytes for Li-ion batteries. Adv. Mater. 31, 1804882 (2019).
Hernandez, G. et al. Elimination of fluorination: the influence of fluorine-free electrolytes on the performance of LiNi1/3Mn1/3Co1/3O2/silicon-graphite Li-ion battery cells. ACS Sustain. Chem. Eng. 8, 10041–10052 (2020).
Christensen, J. & Newman, J. A mathematical model for the lithium-ion negative electrode solid electrolyte interphase. J. Electrochem. Soc. 151, A1977–A1988 (2004).
Gewald, T., Lienkamp, M., Lehmkuhl, D. & Hahn, A. Accelerated aging characterization of lithium-ion cells: limitation of arrhenius dependency. In Fourteenth International Conference on Ecological Vehicles and Renewable Energies 1–10 (IEEE, 2019).
Acknowledgements
This research was supported by the US Department of Energy (DOE)’s Vehicle Technologies Office under the Silicon Consortium Project. This work was conducted in part by the Alliance for Sustainable Energy, LLC, the manager and operator of the National Renewable Energy Laboratory for the DOE under contract no. DE-AC36-08GO28308. The submitted manuscript has been created by UChicago Argonne, LLC, Operator of Argonne National Laboratory (‘Argonne’). Argonne, a US DOE Office of Science laboratory, is operated under contract no. DE-AC02-06CH11357. This manuscript has been authored by UT-Battelle, LLC, under contract no. DE-AC05-00OR22725 with the US DOE. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the US DOE’s National Nuclear Security Administration under contract DE-NA0003525. Lawrence Berkeley National Laboratory is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US DOE under contract no. DE-AC02-05CH11231. The views expressed in the article do not necessarily represent the views of the DOE or the US Government. The US Government retains and the publisher, by accepting the article for publication, acknowledges that the US Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for US Government purposes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Jun-Tao Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McBrayer, J.D., Rodrigues, MT.F., Schulze, M.C. et al. Calendar aging of silicon-containing batteries. Nat Energy 6, 866–872 (2021). https://doi.org/10.1038/s41560-021-00883-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00883-w
This article is cited by
-
High voltage electrolytes for lithium-ion batteries with micro-sized silicon anodes
Nature Communications (2024)
-
Interfacial structure changes between amorphous silicon anode/liquid electrolyte using a highly dense and flat model electrode
Journal of Solid State Electrochemistry (2024)
-
Designing electrolytes and interphases for high-energy lithium batteries
Nature Reviews Chemistry (2023)
-
Revealing the aging process of solid electrolyte interphase on SiOx anode
Nature Communications (2023)
-
SiO-induced thermal instability and interplay between graphite and SiO in graphite/SiO composite anode
Nature Communications (2023)