Abstract
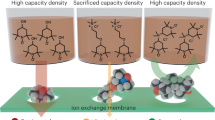
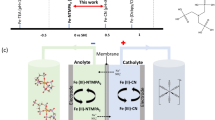
The limited availability of a high-performance catholyte has hindered the development of aqueous organic redox flow batteries (AORFB) for large-scale energy storage. Here we report a symmetry-breaking design of iron complexes with 2,2′-bipyridine-4,4′-dicarboxylic (Dcbpy) acid and cyanide ligands. By introducing two ligands to the metal centre, the complex compounds (M4[FeII(Dcbpy)2(CN)2], M = Na, K) exhibited up to a 4.2 times higher solubility (1.22 M) than that of M4[FeII(Dcbpy)3] and a 50% increase in potential compared with that of ferrocyanide. The AORFBs with 0.1 M Na4[FeII(Dcbpy)2(CN)2] as the catholyte were demonstrated for 6,000 cycles with a capacity fading rate of 0.00158% per cycle (0.217% per day). Even at a concentration near the solubility limit (1 M Na4[FeII(Dcbpy)2(CN)2]), the flow battery exhibited a capacity fading rate of 0.008% per cycle (0.25% per day) in the first 400 cycles. The AORFB cell with a nearly 1:1 catholyte:anolyte electron ratio achieved a cell voltage of 1.2 V and an energy density of 12.5 Wh l–1.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the relevant data are included in the paper and its Supplementary Information. Source data are provided with this paper.
References
Leung, P. et al. Recent developments in organic redox flow batteries: a critical review. J. Power Sources 360, 243–283 (2017).
Soloveichik, G. L. Flow batteries: current status and trends. Chem. Rev. 115, 11533–11558 (2015).
Wang, W. et al. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 23, 970–986 (2013).
Winsberg, J., Hagemann, T., Janoschka, T., Hager, M. D. & Schubert, U. S. Redox-flow batteries: from metals to organic redox-active materials. Angew. Chem. Int. Ed. 56, 686–711 (2017).
Huskinson, B. et al. A metal-free organic-inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Lin, K. et al. Alkaline quinone flow battery. Science 349, 1529–1532 (2015).
Debruler, C., Hu, B., Moss, J., Luo, J. & Liu, T. L. A sulfonate-functionalized viologen enabling neutral cation exchange, aqueous organic redox flow batteries toward renewable energy storage. ACS Energy Lett. 3, 663–668 (2018).
Lin, K. et al. A redox-flow battery with an alloxazine-based organic electrolyte. Nat. Energy 1, 16102 (2016).
Hollas, A. et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries. Nat. Energy 3, 508–514 (2018).
Lai, Y. Y. et al. Stable low-cost organic dye anolyte for aqueous organic redox flow battery. ACS Appl. Energy Mater. 3, 2290–2295 (2020).
Janoschka, T., Martin, N., Hager, M. D. & Schubert, U. S. An aqueous redox-flow battery with high capacity and power: The TEMPTMA/MV system. Angew. Chem. Int. Ed. 55, 14427–14430 (2016).
Liu, T., Wei, X., Nie, Z., Sprenkle, V. & Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte. Adv. Energy Mater. 6, 1501449 (2016).
Hagemann, T. et al. (2,2,6,6-tetramethylpiperidin-1-yl)oxyl-containing zwitterionic polymer as catholyte species for high-capacity aqueous polymer redox flow batteries. Chem. Mater. 31, 7987–7999 (2019).
Janoschka, T. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 527, 78–81 (2015).
Ding, Y., Zhang, C., Zhang, L., Zhou, Y. & Yu, G. Molecular engineering of organic electroactive materials for redox flow batteries. Chem. Soc. Rev. 47, 69–103 (2018).
Wei, X. et al. Materials and systems for organic redox flow batteries: status and challenges. ACS Energy Lett. 2, 2187–2204 (2017).
Kwabi, D. G., Ji, Y. & Aziz, M. J. Electrolyte lifetime in aqueous organic redox flow batteries: a critical review. Chem. Rev. 120, 6467–6489 (2020).
Yang, B. et al. High-performance aqueous organic flow battery with quinone-based redox couples at both electrodes. J. Electrochem. Soc. 163, A1442–A1449 (2016).
Wang, W. Molecular engineering of the redox flow battery technology. In Frontiers in Energy Storage (The Croucher Advanced Study Institute, 2018).
Beh, E. S. et al. A neutral pH aqueous organic–organometallic redox flow battery with extremely high capacity retention. ACS Energy Lett. 2, 639–644 (2017).
Hu, B., Debruler, C., Rhodes, Z. & Liu, T. L. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage. J. Am. Chem. Soc. 139, 1207–1214 (2017).
Waters, S. E., Robb, B. H. & Marshak, M. P. Effect of chelation on iron–chromium redox flow batteries. ACS Energy Lett. 5, 1758–1762 (2020).
Luo, J. et al. Unraveling pH dependent cycling stability of ferricyanide/ferrocyanide in redox flow batteries. Nano Energy 42, 215–221 (2017).
Hosseinzadeh, P. & Lu, Y. Design and fine-tuning redox potentials of metalloproteins involved in electron transfer in bioenergetics. Biochim. Biophys. Acta Bioenerg. 1857, 557–581 (2016).
Lever, A. B. P. Electrochemical parametrization of metal complex redox potentials, using the ruthenium(III)/ruthenium(II) couple to generate a ligand electrochemical series. Inorg. Chem. 29, 1271–1285 (1990).
Kaes, C., Katz, A. & Hosseini, M. W. Bipyridine: the most widely used ligand. A review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 100, 3553–3590 (2000).
Cabrera, P. J. et al. Complexes containing redox noninnocent ligands for symmetric, multielectron transfer nonaqueous redox flow batteries. J. Phys. Chem. C 119, 15882–15889 (2015).
Sevov, C. S., Fisher, S. L., Thompson, L. T. & Sanford, M. S. Mechanism-based development of a low-potential, soluble, and cyclable multielectron anolyte for nonaqueous redox flow batteries. J. Am. Chem. Soc. 138, 15378–15384 (2016).
Gavezzotti, A. Molecular symmetry, melting temperatures and melting enthalpies of substituted benzenes and naphthalenes. J. Chem. Soc. Perkin Trans. 1995, 1399–1404 (1995).
Carnelley, T. XIII. Chemical symmetry, or the influence of atomic arrangement on the physical properties of compounds. Lond. Edinb. Dublin Philos. Mag. 13, 112–130 (1882).
Pinal, R. Effect of molecular symmetry on melting temperature and solubility. Org. Biomol. Chem. 2, 2692–2699 (2004).
Yalkowsky, S. H. & Valvani, S. C. Solubility and partitioning I: solubility of nonelectrolytes in water. J. Pharm. Sci. 69, 912–922 (1980).
Machata, P. et al. Redox reactions of nickel, copper, and cobalt complexes with ‘noninnocent’ dithiolate ligands: combined in situ spectroelectrochemical and theoretical study. Organometallics 33, 4846–4859 (2014).
Bakhtchadjian, R. et al. Application of a dioxo-molybdenum(VI) complex anchored on TiO2 for the photochemical oxidative decomposition of 1-chloro-4-ethylbenzene under O2. Transit. Met. Chem. 36, 897–900 (2011).
Ferrere, S. & Gregg, B. A. Photosensitization of TiO2 by [FeII(2,2′-bipyridine-4,4′-dicarboxylic acid)2(CN)2]: band selective electron injection from ultra-short-lived excited states. J. Am. Chem. Soc. 120, 843–844 (1998).
Luo, J. et al. Unprecedented capacity and stability of ammonium ferrocyanide catholyte in pH neutral aqueous redox flow batteries. Joule 3, 149–163 (2019).
Harris, R. K. et al. Further conventions for NMR shielding and chemical shifts (IUPAC Recommendations 2008). Magn. Reson. Chem. 46, 582–598 (2008).
Han, K. S. et al. Preferential solvation of an asymmetric redox molecule. J. Phys. Chem. C 120, 27834–27839 (2016).
Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 88, 1475–1498 (1988).
Prampolini, G. et al. Structure and dynamics of ferrocyanide and ferricyanide anions in water and heavy water: an insight by MD simulations and 2D IR spectroscopy. J. Phys. Chem. B 118, 14899–14912 (2014).
Daku, L. M. L. & Hauser, A. Ab initio molecular dynamics study of an aqueous solution of [Fe(bpy)3](Cl)2 in the low-spin and in the high-spin states. J. Phys. Chem. Lett. 1, 1830–1835 (2010).
Gierke, T. D., Munn, G. E. & Wilson, F. The morphology in Nafion perfluorinated membrane products, as determined by wide‐and small‐angle X‐ray studies. J. Polym. Sci. B 19, 1687–1704 (1981).
Hsu, W. Y. & Gierke, T. D. Ion transport and clustering in Nafion perfluorinated membranes. J. Membr. Sci. 13, 307–326 (1983).
Mauritz, K. A. & Moore, R. B. State of understanding of Nafion. Chem. Rev. 104, 4535–4586 (2004).
Kwabi, D. G. et al. Alkaline quinone flow battery with long lifetime at pH 12. Joule 2, 1894–1906 (2018).
Koutecky, J. & Levich, B. G. The application of the rotating disc electrode to studies of kinetic and catalytic processes. Zh. Fiz. Khim. 32, 1565–1575 (1958).
Clark, C. D., Debad, J. D., Yonemoto, E. H., Mallouk, T. E. & Bard, A. J. Effect of oxygen on linked Ru(bpy)32+–viologen species and methylviologen: a reinterpretation of the electrogenerated chemiluminescence. J. Am. Chem. Soc. 119, 10525–10531 (1997).
Farrington, J. A., Ledwith, A. & Stam, M. F. Cation–radicals: Oxidation of methoxide ion with 1,1′-dimethyl-4,4′-bipyridylium dichloride (paraquat dichloride). J. Chem. Soc. Chem. Commun. 1969, 259–260 (1969).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 33, 8822–8824 (1986).
Acknowledgements
Y.Z., X.L., Y.-Y.L., W.-Y.T., C.-F.C. Y.-T.L. and H.-Y.L. acknowledge the support of the University of Akron. P.G, J.D.B, A.H., V.M., R.F. and W.W. acknowledge joint financial support from the US Department of Energy (DOE) Office of Electricity (OE) Energy Storage Program (under Contract no. 57558) and Energy Storage Materials Initiative, which is a Laboratory Directed Research and Development Project at Pacific Northwest National Laboratory (PNNL). PNNL is a multi-program national laboratory operated by Battelle for the US DOE under contract DE-AC05-76RL01830.
Author information
Authors and Affiliations
Contributions
X.L, P.G. and Y.-Y.L. contributed equally to the work. X.L. and Y.Z. conceived the idea. W.W. and Y.Z. directed the project. X.L. designed the experiments, performed the synthesis, material characterizations, 1H NMR and 13C NMR spectroscopy, electrochemical measurements and capacity-balanced flow battery tests. P.G. performed the DFT calculations, AIMD simulations and classical MD simulations. Y.-Y.L. fabricated and tested all the catholyte capacity-limiting cells and assisted with materials characterization. J.D.B. performed the 23Na NMR and 17O NMR spectroscopy. A.H. and R.F. verified the solubility and prepared the NMR samples, Y-T.L. and H.-Y.L. assisted with the flow battery fabrication and tests. V.M. oversaw the NMR work. C.-F.C. and R.F. assisted with the electrochemical tests. W.-Y.T., J.W. and S.Z. helped with the materials synthesis and characterization. C.-L.W. helped with the materials design. All the authors discussed and analysed the data. X.L., W.W. and Y.Z. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The metal complex materials disclosed in this work have been filed as US Provisional Patent Application USPTO: 63/080,374 with Y.Z. as the applicant, and Y.Z and X.L. as inventors. The status of the patent application is pending.
Additional information
Peer review information Nature Energy thanks Michael Aziz and Michael Marshak for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–29 and Tables 1 and 2.
Source data
Source Data Fig. 2
NMR source data.
Source Data Fig. 3
Source data for CV, redox potential, RDE and Levich plot.
Source Data Fig. 4
Source data for Ratability test, low concentration cell.
Source Data Fig. 5
Source data for NMR and CV.
Source Data Fig. 6
Source data for high concentration cell.
Rights and permissions
About this article
Cite this article
Li, X., Gao, P., Lai, YY. et al. Symmetry-breaking design of an organic iron complex catholyte for a long cyclability aqueous organic redox flow battery. Nat Energy 6, 873–881 (2021). https://doi.org/10.1038/s41560-021-00879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00879-6
This article is cited by
-
Redox-mediated electrochemical liquid–liquid extraction for selective metal recovery
Nature Chemical Engineering (2024)
-
Phosphonate-based iron complex for a cost-effective and long cycling aqueous iron redox flow battery
Nature Communications (2024)
-
Recent Advances in Redox Flow Batteries Employing Metal Coordination Complexes as Redox-Active Species
Electrochemical Energy Reviews (2024)
-
Modular dimerization of organic radicals for stable and dense flow battery catholyte
Nature Energy (2023)
-
Emerging chemistries and molecular designs for flow batteries
Nature Reviews Chemistry (2022)