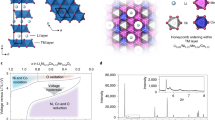
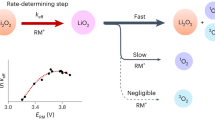
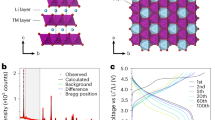
Abstract
The energy density of Li-ion batteries can be improved by storing charge at high voltages through the oxidation of oxide ions in the cathode material. However, oxidation of O2− triggers irreversible structural rearrangements in the bulk and an associated loss of the high voltage plateau, which is replaced by a lower discharge voltage, and a loss of O2 accompanied by densification at the surface. Here we consider various models for oxygen redox that are proposed in the literature and then describe a single unified model involving O2− oxidation to form O2, most of which is trapped in the bulk and the remainder of which evolves from the surface. The model extends the O2 formation and evolution at the surface, which is well known and well characterized, into the electrode particle bulk as caged O2 that can be reversibly reduced and oxidized. This converged understanding enables us to propose practical strategies to avoid oxygen-redox-induced instability and provide potential routes towards more reversible, high energy density Li-ion cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lu, Z., Beaulieu, L. Y., Donaberger, R. A., Thomas, C. L. & Dahn, J. R. Synthesis, structure, and electrochemical behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2. J. Electrochem. Soc. 149, A778 (2002).
Lu, Z. & Dahn, J. R. Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 149, A815 (2002).
Johnson, C. S. et al. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004).
Kim, J.-S. et al. Electrochemical and structural properties of xLi2M′O3·(1−x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M′ = Ti, Mn, Zr; 0 ≤ x ≤ 0.3). Chem. Mater. 16, 1996–2006 (2004).
Koga, H. et al. Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J. Power Sources 236, 250–258 (2013).
Oishi, M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2. J. Power Sources 276, 89–94 (2015).
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Muhammad, S. et al. Evidence of reversible oxygen participation in anomalously high capacity Li- and Mn-rich cathodes for Li-ion batteries. Nano Energy 21, 172–184 (2016).
Gent, W. E. et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 8, 2091 (2017).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
Perez, A. J. et al. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4. Nat. Energy 2, 954–962 (2017).
Hong, J. et al. Metal–oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat. Mater. 18, 256–265 (2019).
Luo, K. et al. Anion redox chemistry in the cobalt free 3d transition metal oxide intercalation electrode Li[Li0.2Ni0.2Mn0.6]O2. J. Am. Chem. Soc. 138, 11211–11218 (2016).
Yabuuchi, N. et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 7, 13814 (2016).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Hansen, C. J. et al. Multielectron, cation and anion redox in lithium-rich iron sulfide cathodes. J. Am. Chem. Soc. 142, 6737–6749 (2020).
Armstrong, A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 128, 8694–8698 (2006).
Koga, H. et al. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J. Electrochem. Soc. 160, A786–A792 (2013).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Tran, N. et al. Mechanisms associated with the “plateau” observed at high voltage for the overlithiated Li1.12(Ni0.425Mn0.425Co0.15)0.88O2 system. Chem. Mater. 20, 4815–4825 (2008).
Weill, F., Tran, N., Martin, N., Croguennec, L. & Delmas, C. Electron diffraction study of the layered Liy(Ni0.425Mn0.425Co0.15)0.88O2 materials reintercalated after two different states of charge. Electrochem. Solid State Lett. 10, A194 (2007).
Ben Yahia, M., Vergnet, J., Saubanère, M. & Doublet, M.-L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 18, 496–502 (2019).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Chen, H. & Islam, M. S. Lithium extraction mechanism in Li-rich Li2MnO3 involving oxygen hole formation and dimerization. Chem. Mater. 28, 6656–6663 (2016).
Gent, W. E., Abate, I. I., Yang, W., Nazar, L. F. & Chueh, W. C. Design rules for high-valent redox in intercalation electrodes. Joule 4, 1369–1397 (2020).
Okubo, M. & Yamada, A. Molecular orbital principles of oxygen-redox battery electrodes. ACS Appl. Mater. Interfaces 9, 36463–36472 (2017).
Radin, M. D., Vinckeviciute, J., Seshadri, R. & Van der Ven, A. Manganese oxidation as the origin of the anomalous capacity of Mn-containing Li-excess cathode materials. Nat. Energy 4, 639–646 (2019).
Maitra, U. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 10, 288–295 (2018).
Taylor, Z. N. et al. Stabilization of O–O bonds by d0 cations in Li4+xNi1−xWO6 (0 ≤ x ≤ 0.25) rock salt oxides as the origin of large voltage hysteresis. J. Am. Chem. Soc. 141, 7333–7346 (2019).
Xu, J. et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 9, 947 (2018).
House, R. A. et al. First cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk. Nat. Energy 5, 777–785 (2020).
Meng, Y. et al. Inelastic X-ray scattering of dense solid oxygen: evidence for intermolecular bonding. Proc. Natl Acad. Sci. USA 105, 11640–11644 (2008).
Croy, J. R. et al. First-charge instabilities of layered-layered lithium-ion-battery materials. Phys. Chem. Chem. Phys. 17, 24382–24391 (2015).
Mohanty, D. et al. Correlating cation ordering and voltage fade in a lithium–manganese-rich lithium-ion battery cathode oxide: a joint magnetic susceptibility and TEM study. Phys. Chem. Chem. Phys. 15, 19496–19509 (2013).
Croy, J. R., Balasubramanian, M., Gallagher, K. G. & Burrell, A. K. Review of the US Department of Energy’s “Deep Dive” effort to understand voltage fade in Li- and Mn-rich cathodes. Acc. Chem. Res. 48, 2813–2821 (2015).
Dai, K. et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 518–541 (2019).
Yan, P. et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat. Nanotechnol. 14, 602–608 (2019).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Singer, A. et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat. Energy 3, 641–647 (2018).
Qiu, B. et al. Metastability and reversibility of anionic redox-based cathode for high-energy rechargeable batteries. Cell Rep. Phys. Sci. 1, 100028 (2020).
House, R. A. et al. Lithium manganese oxyfluoride as a new cathode material exhibiting oxygen redox. Energy Environ. Sci. 11, 926–932 (2018).
Ji, H. et al. Ultrahigh power and energy density in partially ordered lithium-ion cathode materials. Nat. Energy 5, 213–221 (2020).
Rana, J. et al. Quantifying the capacity contributions during activation of Li2MnO3. ACS Energy Lett. 5, 634–641 (2020).
Guerrini, N. et al. Charging mechanism of Li2MnO3. Chem. Mater. 32, 3733–3740 (2020).
Boivin, E. et al. The role of Ni and Co in suppressing O‐Loss in Li‐rich layered cathodes. Adv. Funct. Mater. 31, 2003660 (2021).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2016).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Sharpe, R. et al. Redox chemistry and the role of trapped molecular O2 in Li-rich disordered rocksalt oxyfluoride cathodes. J. Am. Chem. Soc. 142, 21799–21809 (2020).
Zhu, Z. et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 4, 1049–1058 (2019).
Qiu, B. et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 7, 12108 (2016).
Arhammar, C. et al. Unveiling the complex electronic structure of amorphous metal oxides. Proc. Natl Acad. Sci. USA 108, 6355–6360 (2011).
Acknowledgements
P.G.B. is indebted to the Engineering and Physical Sciences Research Council (EPSRC), Henry Royce Institute for Advanced Materials (under grant IDs EP/R00661X/1, EP/S019367/1, EP/R010145/1) and the Faraday Institution Next Generation Li-ion Cathodes project CATMAT (under grant ID FIRG016) for financial support.
Author information
Authors and Affiliations
Contributions
The manuscript was written by R.A.H., J.-J.M. and P.G.B. with contributions and revisions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Yong Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
House, R.A., Marie, JJ., Pérez-Osorio, M.A. et al. The role of O2 in O-redox cathodes for Li-ion batteries. Nat Energy 6, 781–789 (2021). https://doi.org/10.1038/s41560-021-00780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00780-2
This article is cited by
-
Promoting high-voltage stability through local lattice distortion of halide solid electrolytes
Nature Communications (2024)
-
Trapped O2 and the origin of voltage fade in layered Li-rich cathodes
Nature Materials (2024)
-
Efficient direct repairing of lithium- and manganese-rich cathodes by concentrated solar radiation
Nature Communications (2024)
-
Highly structural stability from small-sized Li2MnO3-like domains in Co-free Li-rich layered oxide cathodes
Journal of Solid State Electrochemistry (2024)
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)