Abstract
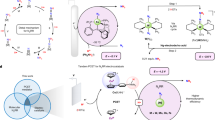
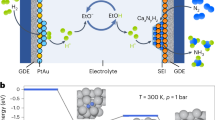
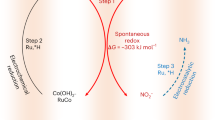
Ammonia (NH3) is essential for modern agriculture and industry and is a potential energy carrier. NH3 is traditionally synthesized by the Haber–Bosch process at high temperature and pressure. The high-energy input of this process has motivated research into electrochemical NH3 synthesis via nitrogen (N2)–water reactions under ambient conditions. However, the future of this low-cost process is compromised by the low yield rate and poor selectivity, ascribed to the inert N≡N bond and ultralow solubility of N2. Obtaining NH3 directly from non-N2 sources could circumvent these challenges. Here we report the eight-electron direct electroreduction of nitrate to NH3 catalysed by copper-incorporated crystalline 3,4,9,10-perylenetetracarboxylic dianhydride. The catalyst exhibits an NH3 production rate of 436 ± 85 μg h−1 cm−2 and a maximum Faradaic efficiency of 85.9% at −0.4 V versus a reversible hydrogen electrode. This notable performance is achieved by the catalyst regulating the transfer of protons and/or electrons to the copper centres and suppressing hydrogen production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available in the paper and Supplementary Information. Additional datasets related to this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Christensen, C. H., Johannessen, T., Sørensen, R. Z. & Nørskov, J. K. Towards an ammonia-mediated hydrogen economy? Catal. Today 111, 140–144 (2006).
Lan, R., Irvine, J. T. & Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrog. Energy 37, 1482–1494 (2012).
Licht, S. et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 345, 637–640 (2014).
van der Ham, C. J., Koper, M. T. & Hetterscheid, D. G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 43, 5183–5191 (2014).
Foster, S. L. et al. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1, 490–500 (2018).
Chen, G. F. et al. Ammonia electrosynthesis with high selectivity under ambient conditions via a Li+ incorporation strategy. J. Am. Chem. Soc. 139, 9771–9774 (2017).
Suryanto, B. H. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Stirling, A., Pápai, I., Mink, J. & Salahub, D. R. Density functional study of nitrogen oxides. J. Chem. Phys. 100, 2910–2923 (1994).
Menció, A. et al. Nitrate pollution of groundwater; all right,… but nothing else? Sci. Total Environ. 539, 241–251 (2016).
Garcia-Segura, S., Lanzarini-Lopes, M., Hristovski, K. & Westerhoff, P. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl. Catal. B 236, 546–568 (2018).
Hirakawa, H., Hashimoto, M., Shiraishi, Y. & Hirai, T. Selective nitrate-to-ammonia transformation on surface defects of titanium dioxide photocatalysts. ACS Catal. 7, 3713–3720 (2017).
Ren, H. T., Jia, S. Y., Zou, J. J., Wu, S. H. & Han, X. A facile preparation of Ag2O/P25 photocatalyst for selective reduction of nitrate. Appl. Catal. B 176, 53–61 (2015).
Dima, G. E., De Vooys, A. C. A. & Koper, M. T. M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 554, 15–23 (2003).
De Vooys, A. C. A., Van Santen, R. A. & Van Veen, J. A. R. Electrocatalytic reduction of NO3– on palladium/copper electrodes. J. Mol. Catal. A 154, 203–215 (2000).
Chen, T., Li, H., Ma, H. & Koper, M. T. M. Surface modification of Pt(100) for electrocatalytic nitrate reduction to dinitrogen in alkaline solution. Langmuir 31, 3277–3281 (2015).
Pérez-Gallent, E., Figueiredo, M. C., Katsounaros, I. & Koper, M. T. Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim. Acta 227, 77–84 (2017).
Ford, C. L., Park, Y. J., Matson, E. M., Gordon, Z. & Fout, A. R. A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 354, 741–743 (2016).
Zheng, H., Wisedchaisri, G. & Gonen, T. Crystal structure of a nitrate/nitrite exchanger. Nature 497, 647–651 (2013).
Oosterkamp, M. J., Mehboob, F., Schraa, G., Plugge, C. M. & Stams, A. J. Nitrate and (per)chlorate reduction pathways in (per)chlorate-reducing bacteria. Biochem. Soc. Trans. 39, 230–235 (2011).
Seefeldt, L. C., Hoffman, B. M. & Dean, D. R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 78, 701–722 (2009).
Khomutov, N. E. & Stamkulov, U. S. Nitrate reduction at various metal electrodes. Sov. Electrochem. 7, 312–316 (1971).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570, 504–508 (2019).
Tsuneto, A., Kudo, A. & Sakata, T. Efficient electrochemical reduction of N2 to NH3 catalyzed by lithium. Chem. Lett. 22, 851–854 (1993).
Gayen, P. et al. Electrocatalytic reduction of nitrate using Magnéli phase TiO2 reactive electrochemical membranes doped with Pd-based catalysts. Environ. Sci. Technol. 52, 9370–9379 (2018).
Machida, M., Sato, K., Ishibashi, I., Hasnat, M. A. & Ikeue, K. Electrocatalytic nitrate hydrogenation over an H+-conducting solid polymer electrolyte membrane-modified cathode assembly. Chem. Commun. 7, 732–734 (2006).
Chaplin, B. P., Shapley, J. R. & Werth, C. J. The selectivity and sustainability of a Pd–In/γ-Al2O3 catalyst in a packed-bed reactor: the effect of solution composition. Catal. Lett. 130, 56–62 (2009).
Hasnat, M. A., Karim, M. R. & Machida, M. Electrocatalytic ammonia synthesis: role of cathode materials and reactor configuration. Catal. Commun. 10, 1975–1979 (2009).
Mo, Z. et al. Electrochemical recognition for tryptophan enantiomers based on 3,4,9,10-perylenetetracarboxylic acid–chitosan composite film. J. Solid State Electrochem. 22, 2405–2412 (2018).
Luo, W., Allen, M., Raju, V. & Ji, X. An organic pigment as a high-performance cathode for sodium-ion batteries. Adv. Energy Mater. 4, 1400554 (2014).
Park, J. Y., Jung, Y. S., Cho, J. & Choi, W. K. Chemical reaction of sputtered Cu film with PI modified by low energy reactive atomic beam. Appl. Surf. Sci. 252, 5877–5891 (2006).
Zhu, D., Zhang, L., Ruther, R. E. & Hamers, R. J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 12, 836–841 (2013).
Green, L. C. et al. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 126, 131–138 (1982).
Watt, G. W. & Chrisp, J. D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 24, 2006–2008 (1952).
Segall, M. D. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 14, 2717–2744 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hamann, D. R., Schlüter, M. & Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 43, 1494–1497 (1979).
Bajdich, M., García-Mota, M., Vojvodic, A., Nørskov, J. K. & Bell, A. T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 135, 13521–13530 (2013).
Acknowledgements
G.-F.C. thanks the Alexander von Humboldt Foundation for a postdoctoral fellowship. This work was financially supported by the National Natural Science Foundation of China (21536005, 51621001 and 21776099), the Post-Doctoral Innovative Talents Project (BX20190119) and National Key R&D Program (2016YFA0202601). A portion of this work was conducted at Argonne National Laboratory. Argonne National Laboratory is operated for the DOE Office of Science by UChicago Argonne, LLC, under contract no. DE-AC02-06CH11357. Use of the Advanced Photon Source (beamline 9BM), Office of Science user facilities, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
G.-F.C. conducted most of experiments. Y.Y. analysed the microscopic and spectroscopic data. L.M. and T.W. conducted the X-ray absorption measurements. G.-F.C., Y.Y., J.L. and H.W. conceived the idea and designed the experiments. G.-F.C., Y.Y., S.-Y.R., H.J., L.-X.D., J.L. and H.W. analysed the data and interpreted the results. S.-Y.R., H.J. and L.-X.D. participated in discussions and data analysis. J.L. and H.W. supervised the project. G.-F.C. and Y.Y. co-wrote the manuscript. All the authors contributed to discussions and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42, Notes 1–7, Supplementary Tables 1–3 and refs. 1–9.
Supplementary Data 1
Replicates data for Supplementary Figs. 2, 5–12, 14 and 36–42.
Source data
Source Data Fig. 1
Experimental source data and DFT calculation source data.
Source Data Fig. 2
Experimental source data.
Source Data Fig. 3
Unprocessed TEM and elemental mapping images and experimental source data.
Source Data Fig. 4
DFT calculations source data
Source Data Fig. 5
DFT calculations source data.
Rights and permissions
About this article
Cite this article
Chen, GF., Yuan, Y., Jiang, H. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat Energy 5, 605–613 (2020). https://doi.org/10.1038/s41560-020-0654-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0654-1