Abstract
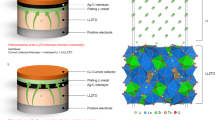
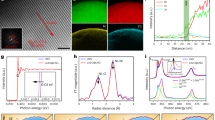
An all-solid-state battery with a lithium metal anode is a strong candidate for surpassing conventional lithium-ion battery capabilities. However, undesirable Li dendrite growth and low Coulombic efficiency impede their practical application. Here we report that a high-performance all-solid-state lithium metal battery with a sulfide electrolyte is enabled by a Ag–C composite anode with no excess Li. We show that the thin Ag–C layer can effectively regulate Li deposition, which leads to a genuinely long electrochemical cyclability. In our full-cell demonstrations, we employed a high-Ni layered oxide cathode with a high specific capacity (>210 mAh g−1) and high areal capacity (>6.8 mAh cm−2) and an argyrodite-type sulfide electrolyte. A warm isostatic pressing technique was also introduced to improve the contact between the electrode and the electrolyte. A prototype pouch cell (0.6 Ah) thus prepared exhibited a high energy density (>900 Wh l−1), stable Coulombic efficiency over 99.8% and long cycle life (1,000 times).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data generated or analysed during this study are included in this published article and its Supplementary Information files. The data that support the plots within this paper are available from the corresponding authors upon reasonable request.
Change history
20 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41560-020-0604-y
References
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Lau, J. et al. Sulfide solid electrolytes for lithium battery applications. Adv. Energy Mater. 8, 1800933 (2018).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Pfenninger, R., Struzik, M., Garbayo, I., Stilp, E. & Rupp, J. L. M. A low ride on processing temperature for fast lithium conduction in garnet solid-state battery films. Nat. Energy 4, 475–483 (2019).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Nie, K. et al. Interfaces between cathode and electrolyte in solid state lithium batteries: challenges and perspectives. Front. Chem. 6, 616 (2018).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 3, 2261 (2013).
Ohtomo, T., Hayashi, A., Tatsumisago, M. & Kawamoto, K. Suppression of H2S gas generation from the 75Li2S·25P2S5 glass electrolyte by additives. J. Mater. Sci. 48, 4137–4142 (2013).
Zhou, L. et al. Solvent-engineered design of argyrodite Li6PS5X (X = Cl, Br, I) solid electrolytes with high ionic conductivity. ACS Energy Lett. 4, 265–270 (2019).
Wenzel, S., Sedlmaier, S. J., Dietrich, C., Zeier, W. G. & Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018).
Chen, B., Xu, C., Wang, H. & Zhou, J. Insights into interfacial stability of Li6PS5Cl solid electrolytes with buffer layers. Curr. Appl. Phys. 19, 149–154 (2019).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Ferrese, A. & Newman, J. Mechanical deformation of a lithium-metal anode due to a very stiff separator. J. Electrochem. Soc. 161, A1350–A1359 (2014).
Masias, A., Felten, N., Garcia-Mendez, R., Wolfenstine, J. & Sakamoto, J. Elastic, plastic, and creep mechanical properties of lithium metal. J. Mater. Sci. 54, 2585–2600 (2019).
Narayan, S. & Anand, L. A large deformation elastic–viscoplastic model for lithium. Extreme Mech. Lett. 24, 21–29 (2018).
Qian, J. et al. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 26, 7094–7102 (2016).
Genovese, M., Louli, A. J., Weber, R., Hames, S. & Dahn, J. R. Measuring the Coulombic efficiency of lithium metal cycling in anode-free lithium metal batteries. J. Electrochem. Soc. 165, A3321–A3325 (2018).
Yang, C.-P., Yin, Y.-X., Zhang, S.-F., Li, N.-W. & Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015).
Weber, R. et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Wang, H. et al. Wrinkled graphene cages as hosts for high-capacity Li metal anodes shown by cryogenic electron microscopy. Nano Lett. 19, 1326–1335 (2019).
Yang, C. et al. Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Adv. Mater. 29, 1702714 (2017).
Park, C.-M., Kim, J.-H., Kim, H. & Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 39, 3115–3141 (2010).
Park, C.-M., Jung, H. & Sohn, H.-J. Electrochemical behaviors and reaction mechanism of nanosilver with lithium. Electrochem. Solid-State Lett. 12, A171–A175 (2009).
Feng, W., Dong, X., Li, P., Wang, Y. & Xia, Y. Interfacial modification of Li/garnet electrolyte by a lithiophilic and breathing interlayer. J. Power Sources 419, 91–98 (2019).
Pei, A., Zheng, G., Shi, F., Li, Y. & Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 17, 1132–1139 (2017).
Wang, M. et al. Porous carbon hosts for lithium–sulfur batteries. Chem. Eur. J. 25, 3710–3725 (2019).
Xie, J. et al. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 4, eaat5168 (2018).
Kraft, M. A. et al. Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1–xGexS5I for all-solid-state batteries. J. Am. Chem. Soc. 140, 16330–16339 (2018).
Kato, Y. et al. All-solid-state batteries with thick electrode configurations. J. Phys. Chem. Lett. 9, 607–613 (2018).
Koerver, R. et al. Redox-active cathode interphases in solid-state batteries. J. Mater. Chem. A 5, 22750–22760 (2017).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Xiao, Y., Miara, L. J., Wang, Y. & Ceder, G. Computational screening of cathode coatings for solid-state batteries. Joule 3, 1252–1275 (2019).
Ito, S. et al. A rocking chair type all-solid-state lithium ion battery adopting Li2O–ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J. Power Sources 248, 943–950 (2014).
Stevens, D. A. & Dahn, J. R. The mechanisms of lithium and sodium insertion in carbon materials. J. Electrochem. Soc. 148, A803–A811 (2001).
Dahn, J. R., Zheng, T., Liu, Y. & Xue, J. S. Mechanisms for lithium insertion in carbonaceous materials. Science 270, 590–593 (1995).
Tachikawa, H. & Shimizu, A. Diffusion dynamics of the Li atom on amorphous carbon: a direct molecular orbital−molecular dynamics study. J. Phys. Chem. B 110, 20445–20450 (2016).
Persson, K. et al. Lithium diffusion in graphitic carbon. J. Phys. Chem. Lett. 1, 1176–1180 (2010).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 9, 618–623 (2014).
Zhang, W. et al. (Electro)chemical expansion during cycling: monitoring the pressure changes in operating solid-state lithium batteries. J. Mater. Chem. A 5, 9929–9936 (2017).
Walther, F. et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite-based all-solid-state batteries using time-of-flight secondary-ion mass spectrometry. Chem. Mater. 31, 3745–3755 (2019).
Hippauf, F. et al. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 21, 390–398 (2019).
Suzuki, N., Yashiro, N., Yamada, T. & Aihara, Y. All-solid-state secondary battery and method of charging the same. US patent no. US20190157723A1.
Suzuki, N., Yashiro, N. & Aihara, Y. Li electroplating/stripping on the metal substrate coated with carbon black. In. Proc. 235th ECS Meeting abstr. 163 (ECS, 2019).
Acknowledgements
This work was supported by funds from Samsung Electronics Co. Ltd.
Author information
Authors and Affiliations
Contributions
D.I., Y.A. and I.T.H. proposed and supervised the research. N.S., N.Y. and Y.A. proposed the metal–carbon composite concept. Y.-G.L., T. Sugimoto, S.R., N.S. and N.Y. optimized the Ag–C composite anode as well as the current collectors for the anode to achieve the best cycle performance. J.H.K., S.F. and T. Shiratsuchi optimized the effect of external pressure on the battery performance. R.O. developed and optimized the SSE. S.F. and T. Shiratsuchi designed and fabricated the high-Ni-based NMC cathode and prototype ASSB as well as the stacked cell (<5 Ah). S.F., R.O. and T. Shiratsuchi carried out the safety tests for LIB and ASSB. C.J. and Y.-G.L. conducted the SEM, Raman and XRD characterizations. D.-S.K. performed the TEM characterization to monitor the morphological changes in the Ag and C particles. S.R. and Y.-G.L. performed the X-ray CT analysis. Y.-G.L., T. Sugimoto, J.H.K., S.R., Y.P., S.F., N.S., N.Y., R.O., T. Shiratsuchi and T.W. performed the electrochemical characterizations. Y.-G.L., D.I., Y.A., S.F. and T.W. analysed the data and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
All the authors are employed at Samsung Electronics Co. Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Notes 1–4 and Tables 1–3.
Supplementary Video 1a
Heating test (LIB)
Supplementary Video 1b
Heating test (ASSB)
Supplementary Video 2
Oil bath test
Supplementary Video 3
Cutting test
Rights and permissions
About this article
Cite this article
Lee, YG., Fujiki, S., Jung, C. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat Energy 5, 299–308 (2020). https://doi.org/10.1038/s41560-020-0575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0575-z
This article is cited by
-
Recycling of solid-state batteries
Nature Energy (2024)
-
A solid-state lithium-ion battery with micron-sized silicon anode operating free from external pressure
Nature Communications (2024)
-
Overcoming low initial coulombic efficiencies of Si anodes through prelithiation in all-solid-state batteries
Nature Communications (2024)
-
Fast cycling of lithium metal in solid-state batteries by constriction-susceptible anode materials
Nature Materials (2024)
-
Sulfide-based composite solid electrolyte films for all-solid-state batteries
Communications Materials (2024)