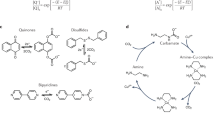
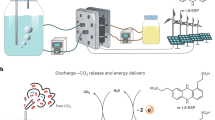
Abstract
CO2 capture technologies based on chemisorption present the potential to lower net emissions of CO2 into the atmosphere. The electrochemical upgrade of captured CO2 to value-added products would be particularly convenient. Here we find that this goal is curtailed when the adduct of the capture molecule with CO2 fails to place the CO2 sufficiently close to the site of the heterogeneous reaction. We investigate tailoring the electrochemical double layer to achieve the valorization of chemisorbed CO2 in an aqueous monoethanolamine electrolyte. We reveal, using electrochemical studies and in situ surface-enhanced Raman spectroscopy, that a smaller double layer distance correlates with improved activity for CO2 to CO from amine solutions. With the aid of an alkali cation and accelerated mass transport by system design—temperature and concentration—we demonstrate amine–CO2 conversion to CO with 72% Faradaic efficiency at 50 mA cm–2.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files. Source data are provided with this paper.
References
Boot-Handford, M. E. et al. Carbon capture and storage update. Energy Environ. Sci. 7, 130–189 (2014).
Wang, Y., Zhao, L., Otto, A., Robinius, M. & Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 114, 650–665 (2017).
Haszeldine, R. S. Carbon capture and storage: how green can black be? Science 325, 1647–1652 (2009).
Rheinhardt, J. H., Singh, P., Tarakeshwar, P. & Buttry, D. A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2, 454–461 (2017).
Schmickler E. S. W. Electrochemical Electron Transfer: from Marcus Theory to Electrocatalysis (John Wiley and Sons, 2008).
Mizen, M. B. & Wrighton, M. S. Reductive addition of CO2 to 9,10‐phenanthrenequinone. J. Electrochem. Soc. 136, 941–946 (1989).
Watkins, J. D. et al. Redox-mediated separation of carbon dioxide from flue gas. Energy Fuels 29, 7508–7515 (2015).
Ranjan, R. et al. Reversible electrochemical trapping of carbon dioxide using 4,4′-bipyridine that does not require thermal activation. J. Phys. Chem. Lett. 6, 4943–4946 (2015).
Singh, P. et al. Electrochemical capture and release of carbon dioxide using a disulfide–thiocarbonate redox cycle. J. Am. Chem. Soc. 139, 1033–1036 (2017).
Appel, A. M., Newell, R., DuBois, D. L. & Rakowski DuBois, M. Concentration of carbon dioxide by electrochemically modulated complexation with a binuclear copper complex. Inorg. Chem. 44, 3046–3056 (2005).
Stern, M. C. & Hatton, T. A. Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration. RSC Adv. 4, 5906–5914 (2014).
Stern, M. C., Simeon, F., Herzog, H. & Hatton, T. A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 6, 2505–2517 (2013).
Chen, L. et al. Electrochemical reduction of carbon dioxide in a monoethanolamine capture medium. ChemSusChem 10, 4109–4118 (2017).
Khurram, A., He, M. & Gallant, B. M. Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture chemistry. Joule 2, 2649–2666 (2018).
Khurram, A., Yan, L., Yin, Y., Zhao, L. & Gallant, B. M. Promoting amine-activated electrochemical CO2 conversion with alkali salts. J. Phys. Chem. C 123, 18222–18231 (2019).
Li, Y. C. et al. CO2 electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Wang, H., Yuan, X. Z. & Li, H. PEM Fuel Cell Diagnostic Tools (Taylor and Francis, 2011).
Ruffo, R. et al. Impedance analysis of Na0.44MnO2 positive electrode for reversible sodium batteries in organic electrolyte. Electrochim. Acta 108, 575–582 (2013).
Mizuno, Y. et al. Impedance spectroscopic study on interfacial ion transfers in cyanide-bridged coordination polymer electrode with organic electrolyte. Electrochim. Acta 63, 139–145 (2012).
Baggetto, L., Niessen, R. A. H. & Notten, P. H. L. On the activation and charge transfer kinetics of evaporated silicon electrode/electrolyte interfaces. Electrochim. Acta 54, 5937–5941 (2009).
Singh, R. K., Kunimatsu, K., Miyatake, K. & Tsuneda, T. Experimental and theoretical infrared spectroscopic study on hydrated Nafion membrane. Macromolecules 49, 6621–6629 (2016).
Zeng, J., Jean, D.-I., Ji, C. & Zou, S. In situ surface-enhanced Raman spectroscopic studies of Nafion adsorption on Au and Pt electrodes. Langmuir 28, 957–964 (2012).
Batista de Carvalho, L. A. E. & Teixeira-Dias, J. J. C. Raman spectra, conformational stability and normal coordinate analysis of ethylmethylamine. J. Raman Spectrosc. 26, 653–661 (1995).
Long, D. A. Infrared and Raman characteristic group frequencies. J. Raman Spectrosc. 35, 905–905 (2004).
Larkin P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation (Elsevier, 2011).
Tseng, C.-L., Chen, Y.-K., Wang, S.-H., Peng, Z.-W. & Lin, J.-L. 2-Ethanolamine on TiO2 investigated by in situ infrared spectroscopy. Adsorption, photochemistry, and its interaction with CO2. J. Phys. Chem. C. 114, 11835–11843 (2010).
Geddes, A. L. & Bottger, G. L. Infrared spectra of silver-ammine complexes. Inorg. Chem. 8, 802–807 (1969).
Miles, M. G. et al. Raman and infrared spectra of isosteric diammine and dimethyl complexes of heavy metals. Normal-coordinate analysis of (X3Y2) 2Z ions and molecules. Inorg. Chem. 7, 1721–1729 (1968).
Plotzker, I. & Exarhos, G. Spectroscopic studies of ammonia reduction of amorphous AgPO3. J. Phys. Chem. 84, 3486–3486 (1980).
Gunathunge, C. M., Ovalle, V. J. & Waegele, M. M. Probing promoting effects of alkali cations on the reduction of CO at the aqueous electrolyte/copper interface. Phys. Chem. Chem. Phys. 19, 30166–30172 (2017).
Strmcnik, D. et al. Effects of Li+, K+, and Ba2+ cations on the ORR at model and high surface area Pt and Au surfaces in alkaline solutions. J. Phys. Chem. Lett. 2, 2733–2736 (2011).
Thorson, M. R., Siil, K. I. & Kenis, P. J. A. Effect of cations on the electrochemical conversion of CO2 to CO. J. Electrochem. Soc. 160, F69–F74 (2013).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Li, J., Li, X., Gunathunge, C. M. & Waegele, M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl Acad. Sci. USA 116, 9220–9229 (2019).
McCrum, I. T., Hickner, M. A. & Janik, M. J. Quaternary ammonium cation specific adsorption on platinum electrodes: a combined experimental and density functional theory study. J. Electrochem. Soc. 165, F114–F121 (2018).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1, 466–472 (2009).
Pérez-Gallent, E., Marcandalli, G., Figueiredo, M. C., Calle-Vallejo, F. & Koper, M. T. M. Structure- and potential-dependent cation effects on CO reduction at copper single-crystal electrodes. J. Am. Chem. Soc. 139, 16412–16419 (2017).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Chattopadhyay, A. & Boxer, S. G. Vibrational Stark effect spectroscopy. J. Am. Chem. Soc. 117, 1449–1450 (1995).
Bagus, P. S., Nelin, C. J., Müller, W., Philpott, M. R. & Seki, H. Field-induced vibrational frequency shifts of CO and CN chemisorbed on Cu(100). Phys. Rev. Lett. 58, 559–562 (1987).
Ge, A. et al. Interfacial structure and electric field probed by in situ electrochemical vibrational stark effect spectroscopy and computational modeling. J. Phys. Chem. C 121, 18674–18682 (2017).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications 2nd edn (Wiley Textbooks, 2000).
Gupta, N., Gattrell, M. & MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 36, 161–172 (2006).
Zhong, H., Fujii, K., Nakano, Y. & Jin, F. Effect of CO2 bubbling into aqueous solutions used for electrochemical reduction of CO2 for energy conversion and storage. J. Phys. Chem. C. 119, 55–61 (2015).
Hatsukade, T., Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 16, 13814–13819 (2014).
Rosen, J. et al. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal. 5, 4293–4299 (2015).
Singh, M. R., Clark, E. L. & Bell, A. T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. Phys. Chem. Chem. Phys. 17, 18924–18936 (2015).
Zhang, Y. et al. Rate-based process modeling study of CO2 capture with aqueous monoethanolamine solution. Ind. Eng. Chem. Res. 48, 9233–9246 (2009).
Lv, B., Guo, B., Zhou, Z. & Jing, G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 49, 10728–10735 (2015).
Zheng, T. et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3, 265–278 (2019).
Bhargava, S. S. et al. System design rules for intensifying the electrochemical reduction of CO2 to CO on Ag nanoparticles. ChemElectroChem 7, 2001–2011 (2020).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Verma, S., Lu, X., Ma, S., Masel, R. I. & Kenis, P. J. A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2018).
Jeong, H.-Y. et al. Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm−2 with single-atom nickel electrocatalysts. J. Mater. Chem. A 7, 10651–10661 (2019).
Li, Y. C. et al. Bipolar membranes inhibit product crossover in CO2 electrolysis cells. Adv. Sustain. Syst. 2, 1700187 (2018).
Dunwell, M., Wang, J., Yan, Y. & Xu, B. Surface enhanced spectroscopic investigations of adsorption of cations on electrochemical interfaces. Phys. Chem. Chem. Phys. 19, 971–975 (2017).
Kudelski, A. & Pettinger, B. Fluctuations of surface-enhanced Raman spectra of CO adsorbed on gold substrates. Chem. Phys. Lett. 383, 76–79 (2004).
Heyes, J., Dunwell, M. & Xu, B. CO2 reduction on Cu at low overpotentials with surface-enhanced in situ spectroscopy. J. Phys. Chem. C 120, 17334–17341 (2016).
Dederichs, F., Friedrich, K. A. & Daum, W. Sum-frequency vibrational spectroscopy of CO adsorption on Pt(111) and Pt(110) electrode surfaces in perchloric acid solution: effects of thin-layer electrolytes in spectroelectrochemistry. J. Phys. Chem. B 104, 6626–6632 (2000).
Chernyshova, I. V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Acknowledgements
We acknowledge the support of the Ontario Research Fund and the Natural Sciences and Engineering Research Council (NSERC) of Canada. This research was supported by the Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017M3D1A1040689).
Author information
Authors and Affiliations
Contributions
G.L. and Y.C.L. contributed to all experimental works, data analysis and manuscript preparation. J.-Y.K. and Y.-C.J. contributed to electrochemical experiments and NMR analysis. T.P. conducted the gas chromatography mass spectrometry analysis with the labelled 13CO2. D.-H.N. and A.S.R. conducted catalyst characterization. F.L. and M.L. contributed to discussions of the mechanism. A.H.I. participated in discussion of the energy analysis. E.H.S. supervised this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Sichao Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–2, Figs. 1–20 and Tables 1–8.
Supplementary Data 1
Supplementary data for Supplementary Tables 1–5 and Supplementary Figs. 1, 3–7, 9–11 and 13–20.
Source data
Source Data Fig. 1
Numerical data for Fig. 1.
Source Data Fig. 3
Numerical data for Fig. 3.
Source Data Fig. 4
Numerical data for Fig. 4.
Source Data Fig. 5
Numerical data for Fig. 5.
Rights and permissions
About this article
Cite this article
Lee, G., Li, Y.C., Kim, JY. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat Energy 6, 46–53 (2021). https://doi.org/10.1038/s41560-020-00735-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-00735-z
This article is cited by
-
Carbon-efficient carbon dioxide electrolysers
Nature Sustainability (2022)
-
Energy comparison of sequential and integrated CO2 capture and electrochemical conversion
Nature Communications (2022)
-
Toward economical application of carbon capture and utilization technology with near-zero carbon emission
Nature Communications (2022)
-
Continuous CO2 electrolysis using a CO2 exsolution-induced flow cell
Nature Energy (2022)
-
Silica-copper catalyst interfaces enable carbon-carbon coupling towards ethylene electrosynthesis
Nature Communications (2021)