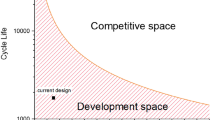
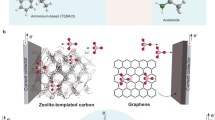
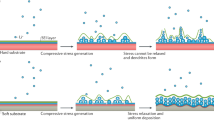
Abstract
Aluminium-based battery technologies have been widely regarded as one of the most attractive options to drastically improve, and possibly replace, existing battery systems—mainly due to the possibility of achieving very high energy density with low cost. Many reports have demonstrated primary or rechargeable Al-based battery chemistries in both aqueous and non-aqueous electrolytes. However, the practical realization of these battery chemistries has been difficult over a long period of time (170 years). In fact, no Al-based battery has been shown with the required stability or touted energy density. Typically, the performance of Al-based batteries is overstated in the literature due to imprecise considerations that do not fairly evaluate practically achievable energy densities. Here we provide accurate calculations of the practically achievable cell-level capacity and energy density for Al-based cells (focusing on recent literature showing ‘high’ performance) and use the results to critically assess their future deployment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
17 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41560-021-00791-z
References
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Ng, B. et al. Low-Temperature lithium plating/corrosion hazard in lithium-ion batteries: electrode rippling, variable states of charge, and thermal and nonthermal runaway. ACS Appl. Energy Mater. 3, 3653–3664 (2020).
Faegh, E. et al. Understanding the dynamics of primary Zn-MnO2 alkaline battery gassing with operando visualization and pressure cells. J. Electrochem. Soc. 165, A2528–A2535 (2018).
Benjamin, P. The Voltaic Cell: Its Construction and Its Capacity (Wiley, 1893).
Heise, G. W., Schumacher, E. A. & Cahoon, N. A heavy duty chlorine‐depolarized cell. J. Electrochem. Soc. 94, 99–105 (1948).
Gifford, P. & Palmisano, J. An aluminum/chlorine rechargeable cell employing a room temperature molten salt electrolyte. J. Electrochem. Soc. 135, 650–654 (1988).
Jayaprakash, N., Das, S. & Archer, L. The rechargeable aluminum-ion battery. Chem. Commun. 47, 12610–12612 (2011).
Ryu, J., Park, M. & Cho, J. Advanced technologies for high‐energy aluminum–air batteries. Adv. Mater. 31, 1804784 (2019).
Ru, Y., Zheng, S., Xue, H. & Pang, H. Different positive electrode materials in organic and aqueous systems for aluminium ion batteries. J. Mater. Chem. A 7, 14391–14418 (2019).
Faegh, E., Shrestha, S., Zhao, X. & Mustain, W. E. In-depth structural understanding of zinc oxide addition to alkaline electrolytes to protect aluminum against corrosion and gassing. J. Appl. Electrochem. 49, 895–907 (2019).
Faegh, E., Ng, B., Hayman, D. & Mustain, W. E. Design of highly reversible zinc anodes for aqueous batteries using preferentially oriented electrolytic zinc. Batter. Supercaps 3, 1220–1232 (2020).
Chen, L. D., Nørskov, J. K. & Luntz, A. C. Al–air batteries: fundamental thermodynamic limitations from first-principles theory. J. Phys. Chem. Lett. 6, 175–179 (2014).
Choi, S. et al. Shape‐reconfigurable aluminum–air batteries. Adv. Funct. Mater. 27, 1702244 (2017).
Yu, Y. et al. Laser sintering of printed anodes for al-air batteries. J. Electrochem. Soc. 165, A584–A592 (2018).
Wang, Y. et al. Liquid-free Al-air batteries with paper-based gel electrolyte: a green energy technology for portable electronics. J. Power Sources 437, 226896 (2019).
Wang, Y. et al. Parametric study and optimization of a low-cost paper-based Al-air battery with corrosion inhibition ability. Appl. Energy 251, 113342 (2019).
Wang, Y. et al. Combining Al-air battery with paper-making industry, a novel type of flexible primary battery technology. Electrochim. Acta 319, 947–957 (2019).
Hopkins, B. J., Shao-Horn, Y. & Hart, D. P. Suppressing corrosion in primary aluminum–air batteries via oil displacement. Science 362, 658–661 (2018).
Yang, H. et al. The rechargeable aluminum battery: opportunities and challenges. Angew. Chem. Int. Ed. 58, 11978–11996 (2019).
Zhang, Y., Liu, S., Ji, Y., Ma, J. & Yu, H. Emerging Nonaqueous Aluminum‐Ion Batteries: Challenges, Status, and Perspectives. Adv. Mater. 30, 1706310 (2018).
Liu, T. et al. An overview and future perspectives of aqueous rechargeable polyvalent ion batteries. Energy Storage Mater. 18, 68–91 (2019).
Bhauriyal, P., Mahata, A. & Pathak, B. The staging mechanism of AlCl 4 intercalation in a graphite electrode for an aluminium-ion battery. Phys. Chem. Chem. Phys. 19, 7980–7989 (2017).
Kravchyk, K. V., Wang, S., Piveteau, L. & Kovalenko, M. V. Efficient aluminum chloride–natural graphite battery. Chem. Mater. 29, 4484–4492 (2017).
Kravchyk, K. V., Seno, C. & Kovalenko, M. V. Limitations of Chloroaluminate Ionic Liquid Anolytes for Aluminum–Graphite Dual-Ion Batteries. ACS Energy Lett. 5, 545–549 (2020).
Elia, G. A., Kyeremateng, N. A., Marquardt, K. & Hahn, R. An aluminum/graphite battery with ultra‐high rate capability. Batter. Supercaps 2, 83–90 (2019).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324 (2015).
Sun, H. et al. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 51, 11892–11895 (2015).
Chen, H. et al. A defect‐free principle for advanced graphene cathode of aluminum‐ion battery. Adv. Mater. 29, 1605958 (2017).
Wang, D.-Y. et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 8, 14283 (2017).
Chen, H. et al. Ultrafast all-climate aluminum-graphene battery with quarter-million cycle life. Sci. Adv. 3, eaao7233 (2017).
Yu, X., Wang, B., Gong, D., Xu, Z. & Lu, B. Graphene nanoribbons on highly porous 3D graphene for high‐capacity and ultrastable Al‐ion batteries. Adv. Mater. 29, 1604118 (2017).
Kim, D. J. et al. Rechargeable aluminium organic batteries. Nat. Energy 4, 51 (2019).
VahidMohammadi, A., Hadjikhani, A., Shahbazmohamadi, S. & Beidaghi, M. Two-dimensional vanadium carbide (MXene) as a high-capacity cathode material for rechargeable aluminum batteries. ACS nano 11, 11135–11144 (2017).
Wang, W. et al. A new cathode material for super-valent battery based on aluminium ion intercalation and deintercalation. Sci. Rep. 3, 3383 (2013).
Yang, W. et al. Flexible free-standing MoS2/carbon nanofibers composite cathode for rechargeable aluminum-ion batteries. ACS Sustain. Chem. Eng. 7, 4861–4867 (2019).
Li, H. et al. A highly reversible Co3S4 microsphere cathode material for aluminum-ion batteries. Nano Energy 56, 100–108 (2019).
Wang, P. et al. A flexible aqueous Al ion rechargeable full battery. Chem. Eng. J. 373, 580–586 (2019).
Wang, S. et al. A novel ultrafast rechargeable multi‐ions battery. Adv. Mater. 29, 1606349 (2017).
Yuan, D., Zhao, J., Manalastas, W. Jr, Kumar, S. & Srinivasan, M. Emerging rechargeable aqueous aluminum ion battery: Status, challenges, and outlooks. Nano Mater. Sci. 2, 248–263 (2020).
Wang, P. et al. A high-performance flexible aqueous Al ion rechargeable battery with long cycle life. Energy Storage Mater. 25, 426–435 (2020).
Wu, C. et al. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat. Commun. 10, 73 (2019).
Zhao, Q. et al. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 4, eaau8131 (2018).
He, S. et al. A high‐energy aqueous aluminum‐manganese battery. Adv. Funct. Mater. 29, 1905228 (2019).
Pan, W. et al. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J. Mater. Chem. A 7, 17420–17425 (2019).
Zu, C.-X. & Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 4, 2614–2624 (2011).
Chao, D. et al. An electrolytic Zn–MnO2 battery for high‐voltage and scalable energy storage. Angew. Chem. In.t Ed. 58, 7823–7828 (2019).
Harlow, J. E. et al. A wide range of testing results on an excellent lithium-ion cell chemistry to be used as benchmarks for new battery technologies. J. Electrochem. Soc. 166, A3031–A3044 (2019).
Gelman, D., Shvartsev, B. & Ein-Eli, Y. Aluminum–air battery based on an ionic liquid electrolyte. J. Mater. Chem. A 2, 20237–20242 (2014).
Hu, Y. et al. A binder‐free and free‐standing cobalt sulfide@ carbon nanotube cathode material for aluminum‐ion batteries. Adv. Mater. 30, 1703824 (2018).
Xu, J. et al. Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)‐ion batteries. Adv. Sci. 4, 1700146 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Stanley Whittingham and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faegh, E., Ng, B., Hayman, D. et al. Practical assessment of the performance of aluminium battery technologies. Nat Energy 6, 21–29 (2021). https://doi.org/10.1038/s41560-020-00728-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-00728-y
This article is cited by
-
Practical assessment of the energy density of potassium-ion batteries
Science China Chemistry (2024)
-
Data-driven search for promising intercalating ions and layered materials for metal-ion batteries
Journal of Materials Science (2024)
-
A solution-to-solid conversion chemistry enables ultrafast-charging and long-lived molten salt aluminium batteries
Nature Communications (2023)
-
Air-Stable Binary Hydrated Eutectic Electrolytes with Unique Solvation Structure for Rechargeable Aluminum-Ion Batteries
Nano-Micro Letters (2023)
-
Advancing battery design based on environmental impacts using an aqueous Al-ion cell as a case study
Scientific Reports (2022)