Abstract
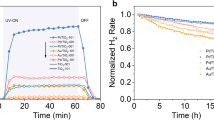
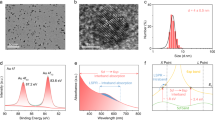
Atomic-level understanding of the active sites and transformation mechanisms under realistic working conditions is a prerequisite for rational design of high-performance photocatalysts. Here, by using correlated scanning fluorescence X-ray microscopy and environmental transmission electron microscopy at atmospheric pressure, in operando, we directly observe that the (110) facet of a single Cu2O photocatalyst particle is photocatalytically active for CO2 reduction to methanol while the (100) facet is inert. The oxidation state of the active sites changes from Cu(i) towards Cu(ii) due to CO2 and H2O co-adsorption and changes back to Cu(i) after CO2 conversion under visible light illumination. The Cu2O photocatalyst oxidizes water as it reduces CO2. Concomitantly, the crystal lattice expands due to CO2 adsorption then reverts after CO2 conversion. The internal quantum yield for unassisted wireless photocatalytic reduction of CO2 to methanol using Cu2O crystals is ~72%.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and the findings of this study are available from the corresponding authors upon reasonable request.
Change history
22 November 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Liu, C. et al. Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures. J. Am. Chem. Soc. 137, 8676–8679 (2015).
Yu, K. M. K., Yeung, C. M. Y. & Tsang, S. C. Carbon dioxide fixation into methyl formate by surface couple over a Pd/Cu/ZnO nanocatalyst. J. Am. Chem. Soc. 129, 6360–6361 (2007).
Liao, F. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH. Angew. Chemie Int. Ed. 50, 2162–2165 (2011).
Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014).
Tang, W. et al. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 14, 76–81 (2012).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Asadi, M. et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 353, 467–470 (2016).
Liu, C., Colon, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Melchionna, M. et al. Pd@TiO2/carbon nanohorn electrocatalysts: reversible CO2 hydrogenation to formic acid. Energy Environ. Sci. 11, 1571–1580 (2018).
Styring, S. Artificial photosynthesis for solar fuels. Faraday Discuss. 155, 357–376 (2012).
Liu, J. et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347, 970–974 (2015).
Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 275, 115–116 (1978).
Tamaki, Y., Morimoto, T., Koike, K. & Ishitani, O. Photocatalytic CO2 reduction with high turnover frequency and selectivity of formic acid formation using Ru(II) multinuclear complexes. Proc. Natl Acad. Sci. USA 109, 15673–15678 (2012).
Sekizawa, Keita, Maeda, Kazuhiko, Domen, Kazunari, Koike, K. & Ishitani, O. Artificial Z scheme constructed with a supramolecular metal complex and semiconductor for the photocatalytic reduction of CO2. J. Am. Chem. Soc. 135, 4596–4599 (2013).
Qiu, J. et al. Artificial photosynthesis on TiO2 -passivated InP nanopillars. Nano Lett. 15, 6177–6181 (2015).
Kothandaraman, J., Goeppert, A., Czaun, M., Olah, Ga & Prakash, G. K. S. Conversion of CO2 from air into methanol using a polyamine and a homogeneous ruthenium catalyst. J. Am. Chem. Soc. 138, 778–781 (2016).
Liu, C. et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 15, 3634–3639 (2015).
Paria, S. & Reiser, O. Copper in photocatalysis. ChemCatChem 6, 2477–2483 (2014).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Angamuthu, R., Byers, P., Lutz, M., Spek, A. L. & Bouwman, E. Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 327, 313–315 (2010).
Reske, R., Mistry, H., Behafarid, F., Cuenya, B. R. & Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 136, 6978–6986 (2014).
Roberts, F. S., Kuhl, K. P. & Nilsson, A. High selectivity for ethylene from carbon dioxide reduction over copper nanocube electrocatalysts Angew. Chemie Int. Ed. 54, 5179–5182 (2015).
Loiudice, A. et al. Tailoring copper nanocrystals towards C2 products in electrochemical CO2 reduction. Angew. Chem. Int. Ed. 55, 5789–5792 (2016).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Paracchino, A., Laporte, V., Sivula, K., Grätzel, M. & Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456–461 (2011).
Kwon, Y., Soon, A., Han, H. & Lee, H. Shape effects of cuprous oxide particles on stability in water and photocatalytic water splitting. J. Mater. Chem. A 3, 156–162 (2015).
Cui, J. & Gibson, U. J. A simple two-step electrodeposition of Cu2O/ZnO nanopillar solar cells. J. Phys. Chem. C 114, 6408–6412 (2010).
Morales, J. et al. Electrodeposition of Cu2O: an excellent method for obtaining films of controlled morphology and good performance in Li-ion batteries. Electrochem. Solid-State Lett. 8, A159–A162 (2005).
Tran, P. D., Wong, L. H., Barber, J. & Loo, J. S. C. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 5, 5902–5918 (2012).
Handoko, A. D. & Tang, J. Controllable proton and CO2 photoreduction over Cu2O with various morphologies. Int. J. Hydrog. Energy 38, 13017–13022 (2013).
An, X., Li, K. & Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 7, 1086–1093 (2014).
Christoforidis, K. C. & Fornasiero, P. Photocatalysis for hydrogen production and CO2 reduction: the case of copper‐catalysts. ChemCatChem 11, 368–382 (2019).
Stolarczyk, J. K., Bhattacharyya, S., Polavarapu, L. & Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 8, 3602–3635 (2018).
Zheng, X. et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 10, 149–154 (2018).
Song, Y. et al. High-selectivity electrochemical conversion of CO2 to ethanol using a copper nanoparticle/n-doped graphene electrode. ChemistrySelect 1, 6055–6061 (2016).
Singh, M. R., Clark, E. L. & Bell, A. T. Thermodynamic and achievable efficiencies for solar-driven electrochemical reduction of carbon dioxide to transportation fuels. Proc. Natl Acad. Sci. USA 112, E6111–E6118 (2015).
Chueh, W. C., Abbott, M., Scipio, D. & Haile, S. M. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330, 1797–1801 (2010).
Koroidov, S., Anderlund, M. F., Styring, S., Thapper, A. & Messinger, J. First turnover analysis of water-oxidation catalyzed by Co-oxide nanoparticles. Energy Environ. Sci. 8, 2492–2503 (2015).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Rajh, T., Ostafin, A. E., Micic, O. I., Tiede, D. M. & Thurnauer, M. C. Surface modification of small particle TiO2 colloids with cysteine for enhanced photochemical reduction: an EPR study. J. Phys. Chem. 100, 4538–4545 (1996).
Wu, Y. A. et al. Visualizing redox dynamics of a single Ag/AgCl heterogeneous nanocatalyst at atomic resolution. ACS Nano 10, 3738–3746 (2016).
Li, Y. et al. Complex structural dynamics of nanocatalysts revealed in operando conditions by correlated imaging and spectroscopy probes. Nat. Commun. 6, 7583 (2015).
Wang, Z. L. New developments in transmission electron microscopy for nanotechnology. Adv. Mater. 15, 1497–1514 (2003).
Yoshida, H. et al. Visualizing gas molecules interacting with supported nanoparticulate catalysts at reaction conditions. Science 335, 317–319 (2012).
Watari, M. et al. Differential stress induced by thiol adsorption on facetted nanocrystals. Nat. Mater. 10, 862–866 (2011).
Symons, M. C. R. Chemical and Biochemical Aspects of Electron-spin Resonance Spectroscopy (Wiley, 1978).
Hulliger, J., Zoller, L. & Ammeter, J. H. Orientation effects in EPR powder samples induced by the static magnetic field. J. Magn. Reson. 48, 512–518 (1982).
Symons, M. C. R., West, D. X. & Wilkinson, J. G. Co-ordination of copper(II) ions doped in pentacyanonitrosylferrate(2-) and tetrathiocyanatometallate(II) salts: an electron spin resonance study. J. Chem. Soc., Dalton Trans. 16, 1696–1700 (1975).
Grechnev, G. E., Savchenko, N. V., Svechkarev, I. V., Lee, M. J. G. & Perz, J. M. Conduction-electron g factors in the noble metals. Phys. Rev. B 39, 9865–9873 (1989).
Liu, C., He, H., Zapol, P. & Curtiss, L. A. Computational studies of electrochemical CO2 reduction on subnanometer transition metal clusters. Phys. Chem. Chem. Phys. 16, 26584–26599 (2014).
Li, R. et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 4, 1432 (2013).
Wang, X. et al. Controlled synthesis of concave Cu2O microcrystals enclosed by {hhl} high-index facets and enhanced catalytic activity. J. Mater. Chem. A 1, 282–287 (2013).
Kuhn, H. J., Braslavsky, S. E. & Schmidt, R. Chemical actinometry (IUPAC Technical Report). Pure Appl. Chem. 76, 2105–2146 (2004).
Demas, J. N., Bowman, W. D., Zalewski, E. F. & Velapoldi, R. A. Determination of the quantum yield of the ferrioxalate actinometer with electrically calibrated radiometers. J. Phys. Chem. 85, 2766–2771 (1981).
Braslavsky, S. E. et al. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 83, 931–1014 (2011).
Jiang, N. Electron beam damage in oxides: a review. Rep. Prog. Phys. 79, 016501 (2016).
Winarski, R. P. et al. A hard X-ray nanoprobe beamline for nanoscale microscopy. J. Synchrotron Rad. 19, 1056–1060 (2012).
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N. & Hausermann, D. Two-dimensional detector software: from real detector to idealised image or two-theta scan. High. Press. Res. 14, 235–248 (1996).
Aoun, B. et al. A generalized method for high throughput in-situ experiment data analysis: an example of battery materials exploration. J. Power Sources 279, 246–251 (2015).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Bendavid, L. I. & Carter, E. A. First-principles predictions of the structure, stability, and photocatalytic potential of Cu2O surfaces. J. Phys. Chem. B 117, 15750–15760 (2013).
Mishra, A. K., Roldan, A. & De Leeuw, N. H. A density functional theory study of the adsorption behaviour of CO2 on Cu2O surfaces. J. Chem. Phys. 145, 044709 (2016).
Acknowledgements
This work was performed at the Center for Nanoscale Materials, a US Department of Energy, Office of Science, Basic Energy Sciences, under Contract no. DE-AC02-06CH11357. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract no. DE-AC02-06CH11357. This work is partially supported by Laboratory Directed Research and Development (LDRD) funding from Argonne National Laboratory, provided by the Director, Office of Science, of the US Department of Energy under Contract no. DE-AC02-06CH11357. The computational studies and X-ray photoemission spectroscopy (XPS) were supported by the US Department of Energy, Basic Energy Sciences, Division of Materials Science and Engineering. C.L., K.C.L. and L.A.C. acknowledge grants of computer time through the LCRC Blues Cluster at Argonne National Laboratory. Cong Liu acknowledges the financial support by US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, under Contract no. DE-AC02-06CH11357. We acknowledge discussions with and the assistance of P. Fuesz, M. Holt, S. Lapidus, E. Rozhkova, C. Fry, R. Zhang, L. Li and M. Chan.
Author information
Authors and Affiliations
Contributions
Y.A.W., I.M., Y.L. and T.R., conceived the ideas. Y.A.W. performed synthesis of samples, ex situ structural characterization, photocatalytic activity characterization, solar-to-fuel efficiency measurements and quantum yield measurements. Y.A.W., Z.C., J.R.G., Y.L. and I.M. performed operando SFXM measurements. Y.A.W. and Y.L. performed operando ETEM, electron diffraction and EELS measurements. Y.A.W. and T.R. performed EPR and isotope tracer measurements. Y.A.W., Q.L. and Y.R. performed operando high-energy X-ray diffraction and ex situ high-resolution X-ray powder diffraction measurements. Y.A.W. and C.-J.S. performed X-ray absorption spectroscopy measurements. A.P.P. and V.S. performed XPS measurements. C.L., K.C.L. and L.A.C. performed DFT computations. Y.A.W., I.M. and T.R. wrote the paper. All authors contributed to the discussion and revision of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23 and Table 1.
Rights and permissions
About this article
Cite this article
Wu, Y.A., McNulty, I., Liu, C. et al. Facet-dependent active sites of a single Cu2O particle photocatalyst for CO2 reduction to methanol. Nat Energy 4, 957–968 (2019). https://doi.org/10.1038/s41560-019-0490-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0490-3
This article is cited by
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
Synthesis of non-cytotoxic Co3O4 nanocatalysts for thermocatalytic methane decomposition by resource recovery
Biomass Conversion and Biorefinery (2024)
-
Optical and microstructural properties of electrodeposited cuprous oxide
Applied Physics A (2024)
-
Electrodeposited Platinum Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application: Enhancement of Photocatalytic Degradation of Amido Black Dye
Catalysis Letters (2024)
-
Polar solvent induced in-situ self-assembly and oxygen vacancies on Bi2MoO6 for enhanced photocatalytic degradation of tetracycline
Nano Research (2024)