Abstract
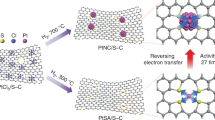
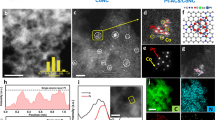
Dispersing catalytically active metals as single atoms on supports represents the ultimate in metal utilization efficiency and is increasingly being used as a strategy to design hydrogen evolution reaction (HER) electrocatalysts. Although platinum (Pt) is highly active for HER, given its high cost it is desirable to find ways to improve performance further while minimizing the Pt loading. Here, we use onion-like nanospheres of carbon (OLC) to anchor stable atomically dispersed Pt to act as a catalyst (Pt1/OLC) for the HER. In acidic media, the performance of the Pt1/OLC catalyst (0.27 wt% Pt) in terms of a low overpotential (38 mV at 10 mA cm−2) and high turnover frequencies (40.78 H2 s−1 at 100 mV) is better than that of a graphene-supported single-atom catalyst with a similar Pt loading, and comparable to a commercial Pt/C catalyst with 20 wt% Pt. First-principle calculations suggest that a tip-enhanced local electric field at the Pt site on the curved support promotes the reaction kinetics for hydrogen evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. B. & Norskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006).
Deng, D. et al. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 11, 218–230 (2016).
Nilsson, A. et al. The electronic structure effect in heterogeneous catalysis. Catal. Lett. 100, 111–114 (2005).
Yin, Y. et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 138, 7965–7972 (2016).
Jiao, Y., Zheng, Y., Davey, K. & Qiao, S. Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 1, 16130 (2016).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–801 (2016).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Yan, H. et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015).
Liu, G. et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 9, 810–816 (2017).
Thomas, J. M. Catalysis: tens of thousands of atoms replaced by one. Nature 525, 325–326 (2015).
Yang, X. F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Bayatsarmadi, B., Zheng, Y., Vasileff, A. & Qiao, S. Z. Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small 13, 1700191 (2017).
Mahmood, J. et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 12, 441–446 (2017).
Cheng, N. et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 7, 13638 (2016).
Tiwari, J. N. et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 3, 773–782 (2018).
Yin, X. P. et al. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 57, 9382–9386 (2018).
Kwak, J. H. et al. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 325, 1670–1673 (2009).
Yang, M. et al. A common single-site Pt(ii)–O(OH)x-species stabilized by sodium on ‘active’ and ‘inert’ supports catalyzes the water–gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Zeiger, M., Jäckel, N., Mochalin, V. N. & Presser, V. Review: carbon onions for electrochemical energy storage. J. Mater. Chem. A 4, 3172–3196 (2016).
Pech, D. et al. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 5, 651–654 (2010).
Ganguly, A., Sharma, S., Papakonstantinou, P. & Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 115, 17009–17019 (2011).
Sun, Z., Liu, Q., Yao, T., Yan, W. & Wei, S. X-ray absorption fine structure spectroscopy in nanomaterials. Sci. China Mater. 58, 313–341 (2015).
Ressler, T. WinXAS: a program for X-ray absorption spectroscopy data analysis under MS-windows. J. Synchrotron Radiat. 5, 118–122 (1998).
Yoshida, H., Nonoyama, S., Yazawa, Y. & Hattori, T. Quantitative determination of platinum oxidation state by XANES analysis. Phys. Scr. T115, 813–815 (2005).
Li, Y. et al. Implementing metal-to-ligand charge transfer in organic semiconductor for improved visible-near-infrared photocatalysis. Adv. Mater. 28, 6959–6965 (2016).
Rennie, A. J., Sanchez-Ramirez, N., Torresi, R. M. & Hall, P. J. Ether-bond-containing ionic liquids as supercapacitor electrolytes. J. Phys. Chem. Lett. 4, 2970–2974 (2013).
Fei, H. et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 6, 8668 (2015).
Zhang, L. et al. Graphene defects trap atomic Ni species for hydrogen and oxygen evolution reactions. Chem 4, 285–297 (2018).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005).
Sundararaman, R., Goddard, W. A. & Arias, T. A. Grand canonical electronic density-functional theory: algorithms and applications to electrochemistry. J. Chem. Phys. 146, 114104 (2017).
Sundararaman, R. et al. JDFTx: software for joint density-functional theory. SoftwareX 6, 278–284 (2017).
Kim, D., Shi, J. & Liu, Y. Substantial impact of charge on electrochemical reactions of two-dimensional materials. J. Am. Chem. Soc. 140, 9127–9131 (2018).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Jiang, H., Hou, Z. & Luo, Y. Unraveling the mechanism for the sharp-tip enhanced electrocatalytic carbon dioxide reduction: the kinetics decide. Angew. Chem. Int. Ed. 56, 15617–15621 (2017).
Oldham, K. B. A Gouy–Chapman–Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 613, 131–138 (2008).
Haleem, Y. A. et al. Surface functionalization and structure characterizations of nanodiamond and its epoxy based nanocomposites. Composites B 78, 480–487 (2015).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Li, Y. et al. An oxygen reduction electrocatalyst based on carbon nanotube–graphene complexes. Nat. Nanotechnol. 7, 394–400 (2012).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Sundararaman, R. & Goddard, W. A. The charge-asymmetric nonlocally determined local-electric (CANDLE) solvation model. J. Chem. Phys. 142, 064107 (2015).
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
Acknowledgements
This study was financially supported in part by the MOST (nos 2017YFA0303500 and 2018YFA0208603), the NSFC (nos 11574280, 11375198, U1532112, 91127042, 21790350, 21633006 and 21473166), the Recruitment Program of Global Experts and the CAS Hundred Talent Program and the Anhui Initiative in Quantum Information Technologies (AHY090200 and AHY090000). We thank the SSRF (14W1), the BSRF (1W1B and soft X-ray), the NSRL (photoemission, MCD and catalysis/surface science) and the USTC Center for Micro and Nanoscale Research and Fabrication for help with the characterization. We also thank Y. Liu and J. Shi for helpful discussions.
Author information
Authors and Affiliations
Contributions
L.S. and J.J. conceived the research and designed the project. D.L. performed most of the experiments. X.L., S.D. and J.J. performed the simulations. S.C. contributed to the XAS measurements. H.Y. and J.L. contributed to the ALD preparation. B.G. contributed to the STEM characterizations. C. Wang, C. Wu and Y.A.H. partially contributed to experimental data. D.L., X.L., P.M.A., Y.L., J.J. and L.S. analysed the data and co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Supplementary Tables 1 and 2, and Supplementary references.
Rights and permissions
About this article
Cite this article
Liu, D., Li, X., Chen, S. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat Energy 4, 512–518 (2019). https://doi.org/10.1038/s41560-019-0402-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0402-6
This article is cited by
-
Lattice oxygen activation and local electric field enhancement by co-doping Fe and F in CoO nanoneedle arrays for industrial electrocatalytic water oxidation
Nature Communications (2024)
-
Pulsed co-electrolysis of carbon dioxide and nitrate for sustainable urea synthesis
Nature Sustainability (2024)
-
Ultra-Efficient and Cost-Effective Platinum Nanomembrane Electrocatalyst for Sustainable Hydrogen Production
Nano-Micro Letters (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Deformable Catalytic Material Derived from Mechanical Flexibility for Hydrogen Evolution Reaction
Nano-Micro Letters (2024)