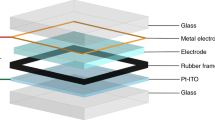
Abstract
Dynamic windows with electronically controlled transmission reduce glare without obstructing views while increasing the energy efficiency of buildings and automobiles via lighting, heating and cooling savings. Electrochromic materials, which change colour with voltage, are widely explored for use in dynamic windows, but they have not been extensively commercialized due to problems associated with colour, cost, switching speed and durability. Here, we develop a class of dynamic windows that combines reversible metal electrodeposition with ion insertion chemistry. These devices function through the reversible electroplating of Bi and Cu at the working electrode and Li+ insertion in a nickel oxide counter electrode. In one minute, 100 cm2 windows uniformly switch between a clear state with 75% transmission and a colour-neutral black state possessing 10% transmission, which represents a significant improvement over previous metal-based architectures. We demonstrate that these hybrid windows cycle at least 4,000 times without degradation and are compatible with flexible substrates. Lastly, we discuss how this approach can be used to design practical large-scale windows.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data that support the plots in this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Lee, E. S., Yazdanian, M. & Selkowitz, S. E. The Energy-savings Potential of Electrochromic Windows in the U.S. Commercial Buildings Sector LBNL-54966 (Lawrence Berkeley National Laboratory, 2004).
Jeffers, M. A., Chaney, L. & Rugh, J. P. Climate Control Load Reduction Strategies for Electric Drive Vehicles in Warm Weather (SAE International, 2015).
Mortimer, R. J., Dyer, A. L. & Reynolds, J. R. Electrochromic organic and polymeric materials for display applications. Displays 27, 2–18 (2006).
Runnerstrom, E. L., Llordes, A., Lounis, S. D. & Milliron, D. J. Nanostructured electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals. Chem. Comm. 50, 10555–10572 (2014).
Granqvist, C. G. Electrochromics for smart windows: oxide-based thin films and devices. Thin Solid Films 564, 1–38 (2014).
Nguyen, W. H., Barile, C. J. & McGehee, M. D. Small molecule anchored to mesoporous ITO for high-contrast black electrochromics. J. Phys. Chem. C 120, 26336–26341 (2016).
Thakur, V. K., Ding, G., Ma, J., Lee, P. S. & Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 24, 4071–4096 (2012).
Barile, C. J. et al. Dynamic windows with neutral color, high contrast, and excellent durability using reversible metal electrodeposition. Joule 1, 133–145 (2017).
Alcaraz, G. K. A., Juarez-Rolon, J. S., Burpee, N. A. & Barile, C. J. Thermally-stable dynamic windows based on reversible metal electrodeposition from aqueous electrolytes. J. Mater. Chem. C 6, 2132–2138 (2018).
Heavens, O. S. Optical Properties of Thin Solid Films (Dover Publications, New York, 1965).
Barile, C. J., Slotcavage, D. J. & McGehee, M. D. Polymer–nanoparticle electrochromic materials that selectively modulate visible and near-infrared light. Chem. Mater. 28, 1439–1445 (2016).
Niklasson, G. A. & Granqvist, C. G. Electrochromics for smart windows: thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 17, 127–156 (2007).
Wen, R. T., Granqvist, C. G. & Niklasson, G. A. Eliminating degradation and uncovering ion-trapping dynamics in electrochromic WO3 thin films. Nat. Mater. 14, 996–1001 (2015).
Gamburg, Y. D. & Zangari, G. Theory and Practice of Metal Electrodeposition (Springer, New York, 2011).
Jensen, J., Madsen, M. V. & Krebs, F. C. Photochemical stability of electrochromic polymers and devices. J. Mater. Chem. C 1, 4826–4835 (2013).
Strand, M. T. et al. Factors that determine the length scale for uniform tinting in dynamic windows based on reversible metal electrodeposition. ACS Energy Lett. 3, 2823–2828 (2018).
Tench, D. Morgan & Warren, L. F. Electrodeposition cell with high light transmission. European patent EP1071980A1 (2001).
Hernandez, T. S. et al. Bistable black electrochromic windows based on the reversible metal electrodeposition of Bi and Cu. ACS Energy Lett. 3, 104–111 (2018).
Lu, Y. et al. Mechanism of electrochemical deposition and coloration of electrochromic V2O5 nano thin films: an in situ X-ray spectroscopy study. Nanoscale Res. Lett. 10, 387–392 (2015).
Varsano, F., Decker, F. & Masetti, E. Optically passive cerium containing counter-electrodes for electrochromic devices. Ionics 5, 80–85 (1999).
Greenwood, N. N. & Earnshaw, A. Chemistry of the Elements (Butterworth-Heinemann, Oxford, 1997).
The Energy Benefits of View Dynamic Glass (View Inc., accessed 30 November 2018); https://view.com/assets/pdfs/workplace-demo-room.pdf
Grujicic, D. & Pesic, B. Electrodeposition of copper: the nucleation mechanisms. Electrochim. Acta 47, 2901–2912 (2002).
Kelly, J. J., Tian, C. & West, A. C. Leveling and microstructural effects of additives for copper electrodeposition. J. Electrochem. Soc. 146, 2540–2545 (1999).
Leung, T. Y. B., Kang, M., Corry, B. F. & Gewirth, A. A. Benzotriazole as an additive for copper electrodeposition influence of triazole ring substitution. J. Electrochem. Soc. 147, 3326–3337 (2000).
Araki, S., Nakamura, K., Kobayashi, K., Tsuboi, A. & Kobayashi, N. Electrochemical optical-modulation device with reversible transformation between transparent, mirror, and black. Adv. Mater. 24, OP122–OP126 (2012).
Passerini, S. & Scrosati, B. The intercalation of lithium in nickel oxide and its electrochromic properties. J. Electrochem. Soc. 137, 3297–3300 (1990).
Wen, R. T., Niklasson, G. A. & Granqvist, C. G. Electrochromic nickel oxide films and their compatibility with potassium hydroxide and lithium perchlorate in propylene carbonate: optical, electrochemical and stress-related properties. Thin Solid Films 565, 128–135 (2014).
Liu, Q. et al. Electrolytes-relevant cyclic durability of nickel oxide thin films as an ion-storage layer in an all-solid-state complementary electrochromic device. Sol. Energy Mat. Sol. C 157, 844–852 (2016).
Mihelčič, M. et al. Comparison of electrochromic properties of Ni1−xO in lithium and lithium-free aprotic electrolytes: from Ni1−xO pigment coatings to flexible electrochromic devices. Sol. Energy Mat. Sol. C 120, 116–130 (2014).
Guo, J. et al. Mechanistic insights into the coloration, evolution, and degradation of NiOx electrochromic anodes. Inorg. Chem. 57, 8874–8880 (2018).
Xia, X. H. et al. Electrochromic properties of porous NiO thin films prepared by a chemical bath deposition. Sol. Energy Mat. Sol. C 92, 628–633 (2008).
Granqvist, C. G. Electrochromic materials: out of a niche. Nat. Mater. 5, 89–90 (2006).
Bergh, H. S. et al. Electrochromic multilayer devices with spatially coordinated switching. US patent US2016/0011481A1 (2016).
Li, Y., Tse, E. C. M., Barile, C. J., Gewirth, A. A. & Zimmerman, S. C. Photoresponsive molecular switch for regulating transmembrane proton-transfer kinetics. J. Am. Chem. Soc. 137, 14059–14062 (2015).
Kondalkar Vijay, V. et al. Electrochromic performance of nickel oxide thin film: synthesis via electrodeposition technique. Macromol. Symp. 361, 47–50 (2016).
Özer, N. Optical properties and electrochromic characterization of sol–gel deposited ceria films. Sol. Energy Mat. Sol. C 68, 391–400 (2001).
Acknowledgements
This research was supported by Research and Innovation at the University of Nevada, Reno and also by the Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Building Energy Efficiency Frontiers in Innovation Technologies Program, Award Number DE-EE0008266/001. T.S.H. acknowledges a National Science Foundation Graduate Research Fellowship (No. NSF DGE-1656518). We acknowledge the Shared Instrumentation Laboratory in the Department of Chemistry at the University of Nevada, Reno (UNR). SEM-EDS analysis was performed in the Mackay Microbeam Laboratory at UNR, and we thank J. Desormeau for his kind assistance. We appreciate R. Kazemi and Dr. Alpuche’s laboratory for assistance in profilometry measurements. We also acknowledge T. Hull for work with CeO2 films, initial large-scale work by J. Jaurez-Rolon, and L. Postak from Quanex for providing the Solargain edge tape. We are grateful for fruitful discussions with M. Strand. We appreciate W. Scheideler, a post-doctoral student in R. Dauskardt’s group at Stanford University, for help with preparing the NiO samples via spray pyrolysis.
Author information
Authors and Affiliations
Contributions
All authors designed the experiments, discussed the results, and commented on the manuscript. S.M.I. and T.S.H. performed the experiments and analysed the data. M.D.M. and C.J.B. wrote the paper. C.J.B. conceived the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figures 1–16
Rights and permissions
About this article
Cite this article
Islam, S.M., Hernandez, T.S., McGehee, M.D. et al. Hybrid dynamic windows using reversible metal electrodeposition and ion insertion. Nat Energy 4, 223–229 (2019). https://doi.org/10.1038/s41560-019-0332-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0332-3
This article is cited by
-
Neutral color and self-healable electrochromic system based on CuI/Cu and \({{\rm{I}}_3}^ - /{{\rm{I}}^ - }\) conversions
Nano Research (2024)
-
Highly transparent silanized cellulose aerogels for boosting energy efficiency of glazing in buildings
Nature Energy (2023)
-
RETRACTED ARTICLE: Enhanced stability of electrochromic devices based on Prussian blue by tuning electrolyte ions and charge/discharge balance
Journal of Applied Electrochemistry (2023)
-
A review and prospect on research progress of adjustable transparent envelope
Building Simulation (2023)
-
Synthesis and applications of Ag@C composites: Progress and opportunity
Journal of Central South University (2022)