Abstract
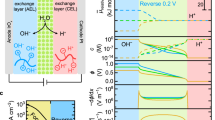
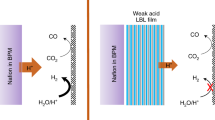
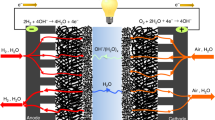
The disparate pH requirements for borohydride oxidation and peroxide reduction in direct borohydride fuel cells (DBFCs) currently hinder their performance and efficiency. Here we develop a pH-gradient-enabled microscale bipolar interface (PMBI) that facilitates sharply different local pH environments at the anode and cathode of a DBFC. Using a recessed planar electrode in conjunction with transmission electron microscopy, we show that the PMBI maintained a sharp local pH gradient (0.82 pH units nm–1 on average) at the electrocatalytic reaction site. The PMBI configuration enabled enhanced performance in a DBFC compared with either all-anion- or all-cation-exchange configurations (330 mA cm–2 at 1.5 V and a peak power density of 630 mW cm–2 at 1.0 V, respectively). The high power densities obtained at voltages well above 1.0 V—achieved by virtue of the effective separation of anolyte and catholyte locally at the electrocatalytically active sites by the PMBI—provide a pathway to reduce fuel cell stack size for autonomous propulsion applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Wang, Z., Parrondo, J. & Ramani, V. Anion exchange membranes based on polystyrene-block-poly(ethylene-ran-butylene)-bock-polystyrene triblock copolymers: cation stability and fuel cell performance. J. Electrochem. Soc. 164, F1216–F1225 (2017).
Parrondo, J., George, M., Capuano, C., Ayers, K. E. & Ramani, V. Pyrochlore electrocatalysts for efficientalkaline water electrolysis.J. Mater. Chem. A 3, 10819–10828 (2015).
Mukerjee, S. & Srinivasan, S. Enhanced electrocatalysis of oxygen reduction on platinum alloys in proton exchange membrane fuel cells. J. Electroanal. Chem. 357, 201–224 (1993).
Marini, S. et al. Advanced alkaline water electrolysis. Electrochim. Acta 82, 384–391 (2012).
Raman, R. K. & Shukla, A. K. A direct borohydride/hydrogen peroxide fuel cell with reduced alkali crossover. Fuel Cells 7, 225–231 (2007).
Ünlü, M., Zhou, J. & Kohl, P. A. Hybrid anion and proton exchange membrane fuel cells. J. Phys. Chem. C 113, 11416–11423 (2009).
Olu, P.-Y., Job, N. & Chatenet, M. Evaluation of anode (electro)catalytic materials for the direct borohydride fuel cell: methods and benchmarks. J. Power Sources 327, 235–257 (2016).
Milikić, J. et al. Pd/c-PANI electrocatalysts for direct borohydride fuel cells. Electrochim. Acta 213, 298–305 (2016).
Liu, B. H., Li, Z. P., Arai, K. & Suda, S. Performance improvement of a micro borohydride fuel cell operating at ambient conditions. Electrochim. Acta 50, 3719–3725 (2005).
Gyenge, E., Atwan, M. & Northwood, D. Electrocatalysis of borohydride oxidation on colloidal Pt and Pt-alloys (Pt-Ir, Pt-Ni, and Pt-Au) and application for direct borohydride fuel cell anodes. J. Electrochem. Soc. 153, A150–A158 (2006).
Park, K. T., Jung, U. H., Jeong, S. U. & Kim, S. H. Influence of anode diffusion layer properties on performance of direct borohydride fuel cell. J. Power Sources 162, 192–197 (2006).
Duteanu, N., Vlachogiannopoulos, G., ShivhareM. R., YuE. H. & Scott, K. A parametric study of a platinum ruthenium anode in a direct borohydride fuel cell. J. Appl. Electrochem. 37, 1085–1091 (2007).
Coowar, F. A., Vitins, G., MepstedG. O.., Waring, S. C. & Horsfall, J. A. Electrochemical oxidation of borohydride at nano-gold-based electrodes: application in direct borohydride fuel cells. J. Power Sources 175, 317–324 (2008).
Qu, C., Zhang, H., Zhang, F. & Liu, B. A high-performance anion exchange membrane based on bi-guanidinium bridged polysilsesquioxane for alkaline fuel cell application. J. Mater. Chem. 22, 8203–8207 (2012).
Arges, C. G., Prabhakaran, V., Wang, L. & Ramani, V. Bipolar polymer electrolyte interfaces for hydrogen-oxygen and direct borohydride fuel cells. Int. J. Hydrogen Energy 39, 14312–14321 (2014).
Liu, B. H., Li, Z. P. & Suda, S. Anodic oxidation of alkali borohydrides catalyzed by nickel. J. Electrochem. Soc. 150, A398–A402 (2003).
Liu, B. H., Li, Z. P. & Suda, S. Electrocatalysts for the anodic oxidation of borohydrides. Electrochim. Acta 49, 3097–3105 (2004).
Çelikkan, H., Şahin, M., Aksu, M. L. & Nejat Veziroğlu, T. The investigation of the electrooxidation of sodium borohydride on various metal electrodes in aqueous basic solutions. Int. J. Hydrogen Energy 32, 588–593 (2007).
Chatenet, M., Micoud, F., Roche, I. & Chainet, E. Kinetics of sodium borohydride direct oxidation and oxygen reduction in sodium hydroxide electrolyte: Part I. BH4 − electro-oxidation on Au and Ag catalysts. Electrochim. Acta 51, 5459–5467 (2006).
Geng, X., Zhang, H., Ma, Y. & Zhong, H. Borohydride electrochemical oxidation on carbon-supported Pt-modified Au nanoparticles. J. Power Sources 195, 1583–1588 (2010).
Finkelstein, D. A. et al. Self-poisoning during BH4 - oxidation at Pt and Au, and in situ poison removal procedures for BH4 - fuel cells. J. Phys. Chem. C 117, 1571–1581 (2013).
Oliveira, V. L., Sibert, E., Soldo-Olivier, Y., Ticianelli, E. A. & Chatenet, M. Borohydride electrooxidation reaction on Pt(111) and Pt(111) modified by a pseudomorphic Pd monolayer. Electrochim. Acta 190, 790–796 (2016).
Oliveira, V. L., Sibert, E., Soldo-Olivier, Y., Ticianelli, E. A. & Chatenet, M. Investigation of the electrochemical oxidation reaction of the borohydride anion in palladium layers on Pt(111). Electrochim. Acta 209, 360–368 (2016).
Braesch, G., Bonnefont, A., Martin, V., Savinova, E. R. & Chatenet, M. Borohydride oxidation reaction mechanisms and poisoning effects on Au, Pt and Pd bulk electrodes: from model (low) to direct borohydride fuel cell operating (high) concentrations. Electrochim. Acta 273, 483–494 (2018).
Olu, P.-Y. et al. Influence of the concentration of borohydride towards hydrogen production and escape for borohydride oxidation reaction on Pt and Au electrodes – experimental and modelling insights. J. Power Sources 375, 300–309 (2018).
Li, Z. P., Liu, B. H., Zhu, J. K. & Suda, S. Depression of hydrogen evolution during operation of a direct borohydride fuel cell. J. Power Sources 163, 555–559 (2006).
Ma, J., Sahai, Y. & Buchheit, R. G. Direct borohydride fuel cell using Ni-based composite anodes. J. Power Sources 195, 4709–4713 (2010).
Gu, L., Luo, N. & Miley, G. H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 173, 77–85 (2007).
Santos, D. M. F., Saturnino, P. G., Lobo, R. F. M. & Sequeira, C. A. C. Direct borohydride/peroxide fuel cells using Prussian blue cathodes. J. Power Sources 208, 131–137 (2012).
He, C., Wang, G., Parrondo, J., Sankarasubramanian, S. & Ramani, V. Pt/RuO2-TiO2 electrocatalysts exhibit excellent hydrogen evolution activity in alkaline media. J. Electrochem. Soc. 164, F1234–F1240 (2017).
Lopez-Haro, M. et al. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 5, 5229 (2014).
Grew, K. N., McClure, J. P., Chu, D., Kohl, P. A. & Ahlfield, J. M. Understanding transport at the aci–alkaline interface of bipolar membranes. J. Electrochem. Soc. 163, F1572–F1587 (2016).
Parrondo, J. et al. Platinum supported on titanium–ruthenium oxide is a remarkably stable electrocatayst for hydrogen fuel cell vehicles. Proc. Natl Acad. Sci. USA 111, 45–50 (2014).
Danilovic, N. et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts.Angew. Chem. Int. Ed. 124, 12663–12666 (2012).
Dinan, T. E., Matlosz, M. & Landolt, D. Experimental investigation of the current distribution on a recessed rotating disk electrode. J. Electrochem. Soc. 138, 2947–2951 (1991).
Haynes W. M. CRC Handbook of Chemistry and Physics (CRC, Boca Raton, 2014).
Atwan, M. H., Macdonald, C. L. B., Northwood, D. O. & Gyenge, E. L. Colloidal Au and Au-alloy catalysts for direct borohydride fuel cells: electrocatalysis and fuel cell performance. J. Power Sources 158, 36–44 (2006).
Cheng, H., Scott, K. & Lovell, K. Material aspects of the design and operation of direct borohydride fuel cells. Fuel Cells 6, 367–375 (2006).
Cheng, H. & Scott, K. Influence of operation conditions on direct borohydride fuel cell performance. J. Power Sources 160, 407–412 (2006).
Cheng, H. & Scott, K. Investigation of Ti mesh-supported anodes for direct borohydride fuel cells. J. Appl. Electrochem. 36, 1361–1366 (2006).
Cheng, H., Scott, K., Lovell, K. V., Horsfall, J. A. & Waring, S. C. Evaluation of new ion exchange membranes for direct borohydride fuel cells. J. Memb. Sci. 288, 168–174 (2007).
Lam, V. W. S. & Gyenge, E. L. High-performance osmium nanoparticle electrocatalyst for direct borohydride PEM fuel cell anodes. J. Electrochem. Soc. 155, B1155–B1160 (2008).
Geng, X., Zhang, H., Ye, W., Ma, Y. & Zhong, H. Ni–Pt/C as anode electrocatalyst for a direct borohydride fuel cell. J. Power Sources 185, 627–632 (2008).
Lam, V. W. S., Alfantazi, A. & Gyenge, E. L. The effect of catalyst support on the performance of PtRu in direct borohydride fuel cell anodes. J. Appl. Electrochem. 39, 1763 (2009).
Celik, C., Boyaci San, F. G. & Sarac, H. I. Improving the direct borohydride fuel cell performance with thiourea as the additive in the sodium borohydride solution. Int. J. Hydrogen Energy 35, 8678–8682 (2010).
Mai, Z., ZhangH., Li, X., Geng, X. & Zhang, H. Polymer electrolyte based on chemically stable and highly conductive alkali-doped polyoxadiazole for direct borohydride fuel cell. Electrochem. Commun. 13, 1009–1012 (2011).
Ma, J., Sahai, Y. & Buchheit, R. G. Evaluation of multivalent phosphate cross-linked chitosan biopolymer membrane for direct borohydride fuel cells. J. Power Sources 202, 18–27 (2012).
Yang, X. et al. A direct borohydride fuel cell with a polymer fiber membrane and non-noble metal catalysts. Sci. Rep. 2, 567 (2012).
Lam, V. W. S., Kannangara, D. C. W., Alfantazi, A. & Gyenge, E. L. Electrodeposited osmium three-dimensional anodes for direct borohydride fuel cells. J. Power Sources 212, 57–65 (2012).
Huang, C.-C. et al. Direct borohydride fuel cell performance using hydroxide-conducting polymeric nanocomposite electrolytes. J. Polym. Sci. B 51, 1779–1789 (2013).
Ma, J. & Sahai, Y. Effect of electrode fabrication method and substrate material on performance of alkaline fuel cells. Electrochem. Commun. 30, 63–66 (2013).
Behmenyar, G. & Akın, A. N. Investigation of carbon supported Pd–Cu nanoparticles as anode catalysts for direct borohydride fuel cell. J. Power Sources 249, 239–246 (2014).
Yang, X., Wei, X., Liu, C. & Liu, Y. The electrocatalytic application of RuO2 in direct borohydride fuel cells. Mater. Chem. Phys. 145, 269–273 (2014).
Boyacı San, F. G., Okur, O., İyigün Karadağ, Ç., Isik-Gulsac, I. & Okumuş, E. Evaluation of operating conditions on DBFC (direct borohydride fuel cell) performance with PtRu anode catalyst by response surface method. Energy 71, 160–169 (2014).
İyigün Karadağ, Ç., Behmenyar, G., Boyacı San, F. G. & Şener, T. Investigation of carbon supported nanostructured PtAu alloy as electrocatalyst for direct borohydride fuel cell. Fuel Cells 15, 262–269 (2015).
Li, G. R., Wang, Q. Q., Liu, B. H. & Li, Z. P. Porous carbon as anode catalyst support to improve borohydride utilization in a direct borohydride fuel cell. Fuel Cells 15, 270–277 (2015).
Olu, P.-Y., Deschamps, F., Caldarella, G., Chatenet, M. & Job, N. Investigation of platinum and palladium as potential anodic catalysts for direct borohydride and ammonia borane fuel cells. J. Power Sources 297, 492–503 (2015).
Zhiani, M. & Mohammadi, I. Performance study of passive and active direct borohydride fuel cell employing a commercial Pd decorated Ni–Co/C anode catalyst. Fuel 166, 517–525 (2016).
Acknowledgements
The authors acknowledge with gratitude the Office of Naval Research (ONR grant no. N00014-16-1-2833) for funding this work. The authors acknowledge the Institute of Materials Science and Engineering for the use of Bruker ICO AFM, JEOL JEM-2000 FX TEM, and staff assistance and the Nano Research Facility within the Department of Energy, Environmental and Chemical Engineering, Washington University in St. Louis for access to SEM facilities. The authors acknowledge financial support from the McKelvery School of Engineering at Washington University in St. Louis. V.R. acknowledges with gratitude generous support from the Roma B. and Raymond H. Wittcoff Distinguished University Professorship.
Author information
Authors and Affiliations
Contributions
Z.W. carried out the DBFC experiments, synthesized the ionomers, characterized the materials by SEM, FTIR, AFM and NMR, analysed the data, prepared the manuscript and aided in revisions. Z.W. and C.H. carried out the RPE experiments. C.H. carried out the TEM experiment. J.P. assisted with the experiments, manuscript revisions and data analysis. S.S. conceived the RPE experiments, carried out some SEM measurements and assisted with the RPE data analysis and manuscript revisions. V.R. conceived and supervised the project and played a primary role in data analysis, manuscript preparation and revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Methods, Supplementary Figures 1–11, Supplementary Tables 1–2, Supplementary References
Rights and permissions
About this article
Cite this article
Wang, Z., Parrondo, J., He, C. et al. Efficient pH-gradient-enabled microscale bipolar interfaces in direct borohydride fuel cells. Nat Energy 4, 281–289 (2019). https://doi.org/10.1038/s41560-019-0330-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0330-5
This article is cited by
-
Preparation and characterization of bimetallic Pd–Zn nanoparticles on carbon for borohydride electrooxidation
Reaction Kinetics, Mechanisms and Catalysis (2021)
-
Oxygen Reduction Reaction on Metal and Nitrogen–Doped Carbon Electrocatalysts in the Presence of Sodium Borohydride
Electrocatalysis (2020)
-
Microwave synthesis of MWCNT-supported PtRuNi catalysts and their electrocatalytic activity for direct methanol fuel cells
Journal of the Korean Ceramic Society (2020)
-
Tailoring membranes
Nature Energy (2019)