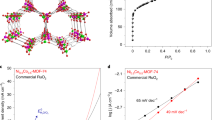
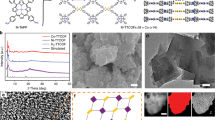
Abstract
Oxygen electrocatalysis is central to technologies such as fuel cells and electrolysers, but challenges remain due to the lack of effective earth-abundant electrocatalysts and insufficient understanding of catalytic mechanisms. Here we demonstrate that robust bifunctional oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activity can be achieved by inducing lattice strain in noble-metal-free metal–organic frameworks (MOFs). Lattice-strained NiFe MOFs exhibit mass activities of 500 A gmetal−1 at a half-wave potential of 0.83 V for the ORR and 2,000 A gmetal−1 at an overpotential of 0.30 V for the OER, which are 50–100 times that of pristine NiFe metal–organic frameworks. The catalyst maintains ~97% of its initial activity after 200 h of continuous ORR/OER reaction at a high current density of 100–200 mA cm−2. Using operando synchrotron spectroscopies, we observed a key superoxide *OOH intermediate emerging on Ni4+ sites during both the ORR and OER processes, which suggests a four-electron mechanistic pathway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Change history
19 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
07 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41560-023-01332-6
References
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater.16, 57–69 (2017).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science355, eaad4998 (2017).
Suen, N. T. et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev.46, 337–365 (2017).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science357, 479–483 (2017).
Guo, D. H. et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science351, 361–365 (2016).
Xia, B. Y. et al. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy1, 15006 (2016).
Yeh, T. F., Teng, C. Y., Chen, S. J. & Teng, H. S. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater.26, 3297–3303 (2014).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science353, 1011–1014 (2016).
Bu, L. Z. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science354, 1410–1414 (2016).
Antolini, E. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells. ACS Catal.4, 1426–1440 (2014).
Huang, J. H. et al. CoOOH nanosheets with high mass activity for water oxidation. Angew. Chem. Int. Ed.54, 8722–8727 (2015).
Chen, P. Z. et al. Atomically dispersed iron–nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew. Chem. Int. Ed.56, 610–614 (2017).
Tse, E. C. M. et al. Proton transfer dynamics control the mechanism of O2 reduction by a non-precious metal electrocatalyst. Nat. Mater.15, 754–759 (2016).
Lee, S., Kapustin, E. A. & Yaghi, O. M. Coordinative alignment of molecules in chiral metal–organic frameworks. Science353, 808–811 (2016).
Sheberla, D. et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater.16, 220–224 (2017).
Zhao, C. M. et al. Ionic exchange of metal organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc.139, 8078–8081 (2017).
Zhao, S. L. et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy1, 16184 (2016).
Chmela, S., Danko, M. & Hrdlovic, P. Preparation, photochemical stability and photostabilizing efficiency of adducts of 1,8-naphthaleneimide and hindered amine stabilizers in polymer matrices. Polym. Degrad. Stab.63, 159–164 (1999).
Fan, L. L. et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun.7, 10667 (2016).
Lu, Z. et al. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun.50, 6479–6482 (2014).
Li, M. F. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science354, 1414–1419 (2016).
Gong, M. et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc.135, 8452–8455 (2013).
Gorlin, M. et al. Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc.138, 5603–5614 (2016).
Yin, P. Q. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed.55, 10800–10805 (2016).
Liu, L. Y., Su, H., Tang, F. M., Zhao, X. & Liu, Q. H. Confined organometallic Au1Nx single-site as an efficient bifunctional oxygen electrocatalyst. Nano Energy46, 110–116 (2018).
Yan, W. S. et al. Valence state-dependent ferromagnetism in Mn-doped NiO thin films. Adv. Mater.24, 353–357 (2012).
Sun, Z. H. et al. Graphene activating room-temperature ferromagnetic exchange in cobalt-doped ZnO dilute magnetic semiconductor quantum dots. ACS Nano8, 10589–10596 (2014).
Petrie, J. R. et al. Enhanced bifunctional oxygen catalysis in strained LaNiO3 perovskites. J. Am. Chem. Soc.138, 2488–2491 (2016).
Liu, J. K. et al. Electron delocalization boosting highly efficient electrocatalytic water oxidation in layered hydrotalcites. J. Phys. Chem. C121, 21962–21968 (2017).
Zhang, M., De Respinis, M. & Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem.6, 362–367 (2014).
Zheng, X. L. et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem.10, 149–154 (2018).
Wang, L. B. et al. Incorporating nitrogen atoms into cobalt nanosheets as a strategy to boost catalytic activity toward CO2 hydrogenation. Nat. Energy2, 869–876 (2017).
Barraclough, C. G., Lawrance, G. A. & Lay, P. A. Characterization of binuclear μ-peroxo and μ-superoxo cobalt(iii) amine complexes from Raman spectroscopy. Inorg. Chem.17, 3317–3322 (1978).
Shibahara, T. & Mori, M. Raman and infrared spectra of μ-O2 dicobalt(iii) complexes. Bull. Chem. Soc. Jpn51, 1374–1379 (1978).
Sivasankar, N., Weare, W. W. & Frei, H. Direct observation of a hydroperoxide surface intermediate upon visible light-driven water oxidation at an Ir oxide nanocluster catalyst by rapid-scan FT-IR spectroscopy. J. Am. Chem. Soc.133, 12976–12979 (2011).
Nakamura, R., Imanishi, A., Murakoshi, K. & Nakato, Y. In situ FTIR studies of primary intermediates of photocatalytic reactions on nanocrystalline TiO2 films in contact with aqueous solutions. J. Am. Chem. Soc.125, 7443–7450 (2003).
Zandi, O. & Hamann, T. W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem.8, 778–783 (2016).
Vanveenendaal, M. A. & Sawatzky, G. A. Doping dependence of Ni 2p X-ray-absorption spectra of MxNi1–xO (M = Li,Na). Phys. Rev. B50, 11326–11331 (1994).
Dey, S. et al. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem.1, 0098 (2017).
Zhu, Y. P., Guo, C. X., Zheng, Y. & Qiao, S. Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res.50, 915–923 (2017).
Pegis, M. L., Wise, C. F., Martin, D. J. & Mayer, J. M. Oxygen reduction by homogeneous molecular catalysts and electrocatalysts. Chem. Rev.118, 2340–2391 (2018).
Brodsky, C. N. et al. In situ characterization of cofacial Co(iv) centers in Co4O4 cubane: modeling the high-valent active site in oxygen-evolving catalysts. Proc. Natl Acad. Sci. USA114, 3855–3860 (2018).
Xiao, H., Shin, H. & Goddard, W. A. III Synergy between Fe and Ni in the optimal performance of (Ni,Fe)OOH catalysts for the oxygen evolution reaction. Proc. Natl Acad. Sci. USA115, 5872–5877 (2018).
Grimaud, A. et al. Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy2, 16189 (2016).
Nellist, M. R. et al. Potential-sensing electrochemical atomic force microscopy for in operando analysis of water-splitting catalysts and interfaces. Nat. Energy3, 46–52 (2018).
Dey, S. et al. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem.1, 0098 (2017).
Zhang, W., Lai, W. Z. & Cao, R. Energy-related small molecule activation reactions: oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev.117, 3717–3797 (2017).
Han, Y. Z., Wu, Y. Z., Lai, W. Z. & Cao, R. Electrocatalytic water oxidation by a water-soluble nickel porphyrin complex at neutral pH with low overpotential. Inorg. Chem.54, 5604–5613 (2015).
Usov, P. M. et al. Cooperative electrochemical water oxidation by Zr nodes and Ni–porphyrin linkers of a PCN-224 MOF thin film. J. Mater. Chem. A4, 16818–16823 (2016).
Acknowledgements
This work was supported by the National Key Research and Development Programme of China (grant nos 2017YFA0402800), the National Natural Science Foundation of China (grant no. U1532265, 11875257, 21603207, 11621063 and 21533007), and the Fundamental Research Funds for the Central Universities (grant nos WK2310000054 and WK2310000070).
Author information
Authors and Affiliations
Contributions
Q.L. and W.Cheng conceived the project. W.Cheng, X.Z. and H.S. carried out the experiments. Q.L., F.T., W.Che and H.Z. analysed the experimental data. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–37, Supplementary Tables 1–3, Supplementary Notes 1–2
Rights and permissions
About this article
Cite this article
Cheng, W., Zhao, X., Su, H. et al. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat Energy 4, 115–122 (2019). https://doi.org/10.1038/s41560-018-0308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0308-8
This article is cited by
-
Tensile straining of iridium sites in manganese oxides for proton-exchange membrane water electrolysers
Nature Communications (2024)
-
Lattice engineered nanoscale Fe0 for selective reductions
Nature Water (2024)
-
Continuous strain tuning of oxygen evolution catalysts with anisotropic thermal expansion
Nature Communications (2024)
-
A restricted dynamic surface self-reconstruction toward high-performance of direct seawater oxidation
Nature Communications (2024)
-
In situ modulating coordination fields of single-atom cobalt catalyst for enhanced oxygen reduction reaction
Nature Communications (2024)