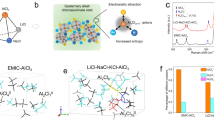
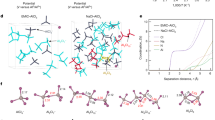
Abstract
Since aluminium is one of the most widely available elements in Earth’s crust, developing rechargeable aluminium batteries offers an ideal opportunity to deliver cells with high energy-to-price ratios. Nevertheless, finding appropriate host electrodes for insertion of aluminium (complex) ions remains a fundamental challenge. Here, we demonstrate a strategy for designing active materials for rechargeable aluminium batteries. This strategy entails the use of redox-active triangular phenanthrenequinone-based macrocycles, which form layered superstructures resulting in the reversible insertion and extraction of a cationic aluminium complex. This architecture exhibits an outstanding electrochemical performance with a reversible capacity of 110 mA h g–1 along with a superior cyclability of up to 5,000 cycles. Furthermore, electrodes composed of these macrocycles blended with graphite flakes result in higher specific capacity, electronic conductivity and areal loading. These findings constitute a major advance in the design of rechargeable aluminium batteries and represent a good starting point for addressing affordable large-scale energy storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Huggins, R. Advanced Batteries: Materials Science Aspects (Springer, 2008).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Tarascon, J.-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Elia, G. A. et al. An overview and future perspectives of aluminum batteries. Adv. Mater. 28, 7564–7579 (2016).
Li, Q. & Bjerrum, N. J. Aluminum as anode for energy storage and conversion: a review. J. Power Sources 110, 1–10 (2002).
Canepa, P. et al. Odyssey of multivalent cathode materials: open questions and future challenges. Chem. Rev. 117, 4287–4341 (2017).
Muldoon, J., Bucur, C. B. & Gregory, T. Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 114, 11683–11720 (2014).
Yoo, D.-J., Kim, J.-S., Shin, J., Kim, K. J. & Choi, J. W. Stable performance of aluminum metal battery by incorporating lithium ion chemistry. ChemElectroChem 4, 2345–2351 (2017).
Dagorne, S. & Atwood, D. A. Synthesis, characterization, and applications of group 13 cationic compounds. Chem. Rev. 108, 4037–4071 (2008).
Atwood, D. A. Cationic group 13 complexes. Coord. Chem. Rev. 176, 407–430 (1998).
Buchanan, R. M. & Pierpont, C. G. Tautomeric catecholate-semiquinone interconversion via metalligand electron-transfer—structural, spectral, and magnetic-properties of (3,5-di-tert-butylcatecholato)-(3,5-di-tert-butylsemiquinone)(bipyridyl)cobalt(III), a complex containing mixed-valence organic-ligands. J. Am. Chem. Soc. 102, 4951–4957 (1980).
Piskunov, A. V., Maleeva, A. V., Fukin, G. K., Baranov, E. V. & Kuznetsova, O. V. Quinone complexes of aluminum: synthesis and structures. Russ. J. Coord. Chem. 36, 161–169 (2010).
Connelly, N. G. & Geiger, W. E. Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Klimov, E. S., Lobanov, A. V. & Abakumov, G. A. Electron-spin-resonance spectra of chelate complexes of 1,2-naphthoquinone and 9,10-phenanthrenequinone with halides of group-III elements. Russ. Chem. Bull. 30, 1664–1666 (1981).
Barker, P. E., Hudson, A. & Jackson, R. A. The reaction of aluminium trichloride with 9,10-phenanthrenequinone. J. Organomet. Chem. 208, C1–C2 (1981).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Hudak, N. S. Chloroaluminate-doped conducting polymers as positive electrodes in rechargeable aluminum batteries. J. Phys. Chem. C 118, 5203–5215 (2014).
Jayaprakash, N., Das, S. K. & Archer, L. A. The rechargeable aluminum-ion battery. Chem. Commun. 47, 12610–12612 (2011).
Zhang, J. et al. Metal-free phenanthrenequinone cyclotrimer as an effective heterogeneous catalyst. J. Am. Chem. Soc. 131, 11296–11297 (2009).
Ohtsuka, Y., Yoshida, J. & Nokami, T., inventors; Panasonic Corporation, assignee. Phenanthrenequinone compound, electrode active material, and power storage device. US patent 12,530,382 (2008).
Tang, L. et al. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 19, 2782–2789 (2009).
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater. 6, 183–191 (2007).
Schwab, M. G. et al. Torands revisited: metal sequestration and self-assembly of cyclo-2,9-tris-1,10-phenanthroline hexaaza macrocycles. Chem. Eur. J. 21, 8426–8434 (2015).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Wang, S. et al. Aluminum chloride-graphite batteries with flexible current collectors prepared from Earth-abundant elements. Adv. Sci. 5, 1700712 (2018).
Hassan, F. M. et al. Evidence of covalent synergy in silicon-sulfur-graphene yielding highly efficient and long-life lithium-ion batteries. Nat. Commun. 6, 8597 (2015).
Kaim, W. Radical-forming electron-transfer reactions involving main-group organometallics. Acc. Chem. Res. 18, 160–166 (1985).
Koten, G. V., Jastrzebski, J. T. B. H. & Vrieze, K. Stable 1,4-diaza-1,3-butadiene(α-diimine)-zinc and -aluminium radicals formed in single electron transfer reactions: their consequences for organic syntheses. J. Organomet. Chem. 250, 49–61 (1983).
Razuvaev, G. A., Abakumov, G. A., Klimov, E. S., Gladyshev, E. N. & Bayushkin, P. Y. Reactions of sterically hindered o-quinones with alkyl derivatives of group III elements. Russ. Chem. Bull. 26, 1034–1037 (1977).
Kravchyk, K. V., Wang, S., Piveteau, L. & Kovalenko, M. V. Efficient aluminum chloride–natural graphite battery. Chem. Mater. 29, 4484–4492 (2017).
Kim, D. J. et al. Redox-active macrocycles for organic rechargeable batteries. J. Am. Chem. Soc. 139, 6635–6643 (2017).
Armand, M. et al. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 8, 120–125 (2009).
Morita, Y. et al. Organic tailored batteries materials using stable open-shell molecules with degenerate frontier orbitals. Nat. Mater. 10, 947–951 (2011).
Lee, M. et al. Organic nanohybrids for fast and sustainable energy storage. Adv. Mater. 26, 2558–2565 (2014).
Liang, Y., Tao, Z. & Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2, 742–769 (2012).
Zhang, Z., Yoshikawa, H. & Awaga, K. Discovery of a “bipolar charging” mechanism in the solid-state electrochemical process of a flexible metal–organic framework. Chem. Mater. 28, 1298–1303 (2016).
Fang, C. et al. A metal–organic compound as cathode material with superhigh capacity achieved by reversible cationic and anionic redox chemistry for high‐energy sodium‐ion batteries. Angew. Chem. Int. Ed. 129, 6897–6901 (2017).
Wang, D.-Y. et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 8, 14283 (2017).
Chen, C. J. et al. Highly conductive, lightweight, low-tortuosity carbon frameworks as ultrathick 3D current collectors. Adv. Energy Mater. 7, 1700595 (2017).
Lee, J. H. et al. Restacking-inhibited 3D reduced graphene oxide for high performance supercapacitor electrodes. ACS Nano 7, 9366–9374 (2013).
Wu, K. H., Wang, D. W. & Gentle, I. R. The value of mixed conduction for oxygen electroreduction on graphene-chitosan composites. Carbon 73, 234–243 (2014).
Acknowledgements
This research was conducted as part of the Joint Center of Excellence in Integrated Nanosystems at King Abdulaziz City for Science and Technology (KACST) and Northwestern University (NU). The authors acknowledge both KACST and NU for their financial support of this research. The Integrated Molecular Structure Education and Research Center (IMSERC) at NU is acknowledged for the use of its facilities. J.W.C. acknowledges support by National Research Foundation of Korea (NRF) grants funded by the Korea government (MEST) (NRF-2018R1A2A1A19023146, NRF-2017M1A2A2044477 and NRF-2018M1A2A2063340) and the Energy Efficiency and Resources Core Technology Programme of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), which is granted financial resources from the Ministry of Trade, Industry and Energy, Republic of Korea (20152020104870).
Author information
Authors and Affiliations
Contributions
D.J.K. and D.-J.Y. designed and performed experimental work. D.J.K., M.T.O., A.P. and M.O. worked on synthesis and characterisation of active materials. D.-J.Y. measured ALB performance. D.-J.Y. and S.J.L. conducted ex-situ analysis of active materials. D.J.K., A.P., D.-J.Y., J.W.C. and J.F.S. wrote the manuscript. J.W.C. and J.F.S. directed this work. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–21, Supplementary Note, Supplementary References
Rights and permissions
About this article
Cite this article
Kim, D.J., Yoo, DJ., Otley, M.T. et al. Rechargeable aluminium organic batteries. Nat Energy 4, 51–59 (2019). https://doi.org/10.1038/s41560-018-0291-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0291-0
This article is cited by
-
Surficial modification enabling planar Al growth toward dendrite-free metal anodes for rechargeable aluminum batteries
Science China Chemistry (2024)
-
Construction of double reaction zones for long-life quasi-solid aluminum-ion batteries by realizing maximum electron transfer
Nature Communications (2023)
-
Additive-Driven Interfacial Engineering of Aluminum Metal Anode for Ultralong Cycling Life
Nano-Micro Letters (2023)
-
Rational Design of Electrode–Electrolyte Interphase and Electrolytes for Rechargeable Proton Batteries
Nano-Micro Letters (2023)
-
Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting
Nature (2022)