Abstract
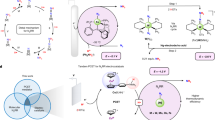
Ammonia is a promising carbon-free energy carrier, but is currently synthesized industrially under harsh conditions. Synthesizing ammonia using lower temperatures and pressures could therefore improve its prospects as a chemical means to store and transport energy. Here we report that alkali and alkaline earth metal imides function as nitrogen carriers that mediate ammonia production via a two-step chemical looping process operating under mild conditions. Nitrogen is first fixed through the reduction of N2 by alkali or alkaline earth metal hydrides to form imides and, subsequently, the imides are hydrogenated to produce NH3 and regenerate the metal hydrides. The oxidation state of hydrogen therefore switches between −1 (hydride), 0 (H2) and +1 (imide and NH3). Late 3d metals accelerate the reaction rates of both steps. The chemical loop mediated by BaNH and catalysed by Ni produces NH3 at 100 °C and atmospheric pressure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon reasonable request.
References
Nørskov, J. & Chen, J. G. Sustainable Ammonia Synthesis (DOE Roundtable Report, 2016).
Philibert, C. Renewable Energy for Industry: From Green Energy to Green Materials and Fuels (IEA Report, 2017).
Guo, J. & Chen, P. Catalyst: NH3 as an energy carrier. Chem 3, 709–712 (2017).
Zhu, D., Zhang, L. H., Ruther, R. E. & Hamers, R. J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 12, 836–841 (2013).
Li, H., Shang, J., Ai, Z. H. & Zhang, L. Z. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J. Am. Chem. Soc. 137, 6393–6399 (2015).
Medford, A. J. & Hatzell, M. C. Photon-driven nitrogen fixation: current progress, thermodynamic considerations, and future outlook. ACS Catal. 7, 2624–2643 (2017).
Licht, S. et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 345, 637–640 (2014).
Chen, S. et al. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew. Chem. Int. Ed. 56, 2699–2703 (2017).
Bao, D. et al. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2 /NH3 cycle. Adv. Mater. 29, 1604799 (2017).
Kyriakou, V., Garagounis, I., Vasileiou, E., Vourros, A. & Stoukides, M. Progress in the electrochemical synthesis of ammonia. Catal. Today 286, 2–13 (2017).
Gálvez, M. E., Halmann, M. & Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. thermodynamic, environmental, and economic analyses. Ind. Eng. Chem. Res. 46, 2042–2046 (2007).
Michalsky, R. & Pfromm, P. H. Chromium as reactant for solar thermochemical synthesis of ammonia from steam, nitrogen, and biomass at atmospheric pressure. Sol. Energy 85, 2642–2654 (2011).
Michalsky, R., Parman, B. J., Amanor-Boadu, V. & Pfromm, P. H. Solar thermochemical production of ammonia from water, air and sunlight: Thermodynamic and economic analyses. Energy 42, 251–260 (2012).
Michalsky, R., Pfromm, P. H. & Steinfeld, A. Rational design of metal nitride redox materials for solar-driven ammonia synthesis. Interface Focus 5, 20140084 (2015).
McEnaney, J. M. et al. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 10, 1621–1630 (2017).
Anheden, M. & Svedberg, G. Exergy analysis of chemical-looping combustion systems. Energy Convers. Manag. 39, 1967–1980 (1998).
Fan, L.-S. Chemical Looping Systems for Fossil Energy Conversions (John Wiley & Sons, Hoboken, 2011).
Adanez, J., Abad, A., Garcia-Labiano, F., Gayan, P. & de Diego, L. F. Progress in chemical-looping combustion and reforming technologies. Prog. Energ. Combust. 38, 215–282 (2012).
Bertuccioli, L. et al. Development of Water Electrolysis in the European Union (Fuel Cells and Hydrogen Joint Undertaking, 2014).
Ertl, G. Surface science and catalysis—studies on the mechanism of ammonia synthesis: the P. H. Emmett Award Address. Catal. Rev. 21, 201–223 (1980).
Schlögl, R. in Handbook of Heterogeneous Catalysis (eds Ertl, G. et al.) Ch. 12.1 (Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, 2008).
Pfromm, P. H. Towards sustainable agriculture: fossil-free ammonia. J. Renew. Sustain. Ener. 9, 034702 (2017).
Jennings, J. R. (ed.) Catalytic Ammonia Synthesis: Fundamentals and Practice (Springer, New York, 1991).
Michalsky, R., Avram, A. M., Peterson, B. A., Pfromm, P. H. & Peterson, A. A. Chemical looping of metal nitride catalysts: low-pressure ammonia synthesis for energy storage. Chem. Sci. 6, 3965–3974 (2015).
Laassiri, S., Zeinalipour-Yazdi, C. D., Catlow, C. R. A. & Hargreaves, J. S. J. The potential of manganese nitride based materials as nitrogen transfer reagents for nitrogen chemical looping. Appl. Catal. B 223, 60–66 (2018).
Chen, P., Xiong, Z., Luo, J., Lin, J. & Tan, K. L. Interaction of hydrogen with metal nitrides and imides. Nature 420, 302–304 (2002).
Grochala, W. & Edwards, P. P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 104, 1283–1316 (2004).
Orimo, S., Nakamori, Y., Eliseo, J. R., Züttel, A. & Jensen, C. M. Complex hydrides for hydrogen storage. Chem. Rev. 107, 4111–4132 (2007).
He, T., Pachfule, P., Wu, H., Xu, Q. & Chen, P. Hydrogen carriers. Nat. Rev. Mater. 1, 16059 (2016).
Gao, W. et al. Barium hydride-mediated nitrogen transfer and hydrogenation for ammonia synthesis: a case study of cobalt. ACS Catal. 7, 3654–3661 (2017).
Ichikawa, T., Isobe, S., Hanada, N. & Fujii, H. Lithium nitride for reversible hydrogen storage. J. Alloy. Compd. 365, 271–276 (2004).
Zhang, J. & Hu, Y. H. Intermediate species and kinetics of lithium imide decomposition. Int. J. Hydrog. Energy 37, 10467–10472 (2012).
David, W. I. F. et al. A mechanism for non-stoichiometry in the lithium amide/lithium imide hydrogen storage reaction. J. Am. Chem. Soc. 129, 1594–1601 (2007).
Verbraeken, M. C., Cheung, C., Suard, E. & Irvine, J. T. S. High H- ionic conductivity in barium hydride. Nat. Mater. 14, 95–100 (2015).
Hagen, S. et al. New efficient catalyst for ammonia synthesis: barium-promoted cobalt on carbon. Chem. Commun. 11, 1206–1207 (2002).
Kojima, R. & Aika, K. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis: Part 2. Kinetic study. Appl. Catal. A 218, 121–128 (2001).
Liu, C. Y. & Aika, K. Ammonia absorption into alkaline earth metal halide mixtures as an ammonia storage material. Ind. Eng. Chem. Res. 43, 7484–7491 (2004).
Sørensen, R. Z. et al. Indirect, reversible high-density hydrogen storage in compact metal ammine salts. J. Am. Chem. Soc. 130, 8660–8668 (2008).
Walker, G. Solid-state Hydrogen Storage: Materials and Chemistry (CRC Press, Boca Raton, 2008).
Aika, K. & Tamaru, K. Ammonia: Catalysis and Manufacture (Springer, Heidelberg, 1995).
Wang, P. K. et al. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem. 9, 64–71 (2017).
Takagi, S. & Orimo, S. Recent progress in hydrogen-rich materials from the perspective of bonding flexibility of hydrogen. Scr. Mater. 109, 1–5 (2015).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Uysal, M., Karslioglu, R., Alp, A. & Akbulut, H. The preparation of core-shell Al2O3/Ni composite powders by electroless plating. Ceram. Int. 39, 5485–5493 (2013).
Aika, K., Takano, T. & Murata, S. Preparation and characterization of chlorine-free ruthenium catalysts and the promoter effect in ammonia-synthesis. 3. A magnesia-supported ruthenium catalyst. J. Catal. 136, 126–140 (1992).
Mckay, D., Gregory, D. H., Hargreaves, J. S. J., Hunter, S. M. & Sun, X. L. Towards nitrogen transfer catalysis: reactive lattice nitrogen in cobalt molybdenum nitride. Chem. Commun. 2007 3051–3053 (2007).
Sato, K. et al. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 8, 674–679 (2017).
Acknowledgements
The authors are grateful for the financial support from National Natural Science Foundation of China (Grant Nos. 21633011 and 21603220), Sino-Japanese Research Cooperative Program of Ministry of Science and Technology (2016YFE0118300), DICP (DICP DMTO201504), Youth Innovation Promotion Association CAS (No. 2018213) and Collaborative Innovation Center of Chemistry for Energy Materials (2011-iChEM).
Author information
Authors and Affiliations
Contributions
P.C. conceived the research and wrote the paper. J.G. coordinated the experimental work. W.G. performed the synthesis, characterization and ammonia production rate measurements of the materials. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Supplementary Figures 1–10, Supplementary Tables 1–4, Supplementary references
Rights and permissions
About this article
Cite this article
Gao, W., Guo, J., Wang, P. et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers. Nat Energy 3, 1067–1075 (2018). https://doi.org/10.1038/s41560-018-0268-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0268-z
This article is cited by
-
Calcium-mediated nitrogen reduction for electrochemical ammonia synthesis
Nature Materials (2024)
-
Photodriven nitrogen fixation by lithium hydride
Nature Chemistry (2024)
-
Light-driven ammonia synthesis under mild conditions using lithium hydride
Nature Chemistry (2024)
-
Anionic Defects Enhanced Ammonia Synthesis Over Ru Catalyst Supported on Barium Niobate Reduced with CaH2
Catalysis Letters (2024)
-
Prospects and challenges of green ammonia synthesis
Nature Synthesis (2023)