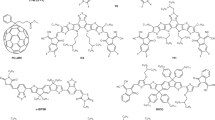
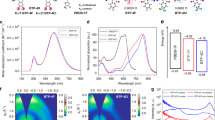
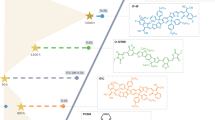
Abstract
The performance of organic photovoltaics is largely dependent on the balance of short-circuit current density (JSC) and open-circuit voltage (VOC). For instance, the reduction of the active materials’ optical bandgap, which increases the JSC, would inevitably lead to a concomitant reduction in VOC. Here, we demonstrate that careful tuning of the chemical structure of photoactive materials can enhance both JSC and VOC simultaneously. Non-fullerene organic photovoltaics based on a well-matched materials combination exhibit a certified high power conversion efficiency of 12.25% on a device area of 1 cm2. By combining Fourier-transform photocurrent spectroscopy and electroluminescence, we show the existence of a low but non-negligible charge transfer state as the possible origin of VOC loss. This study highlights that the reduction of the bandgap to improve the efficiency requires a careful materials design to minimize non-radiative VOC losses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Zhao, J. et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 1, 15027 (2016).
Li, S. et al. Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv. Mater. 28, 9423–9429 (2016).
Zhao, F. et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 29, 1700144 (2017).
Jiang, W. et al. Ternary nonfullerene polymer solar cells with 12.16% efficiency by introducing one acceptor with cascading energy level and complementary absorption. Adv. Mater. 30, 1703005 (2018).
Sun, C. et al. A low cost and high performance polymer donor material for polymer solar cells. Nat. Commun. 9, 743 (2018).
Xu, S. et al. A twisted thieno[3,4-b]thiophene-based electron acceptor featuring a 14-π-electron indenoindene core for high-performance organic photovoltaics. Adv. Mater. 29, 1704510 (2017).
Fan, Q. et al. Synergistic effect of fluorination on both donor and acceptor materials for high performance non-fullerene polymer solar cells with 13.5% efficiency. Sci. China Chem. 61, 531–537 (2018).
Xiao, Z., Jia, X. & Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 62, 1562–1564 (2017).
Xu, X. et al. Highly efficient ternary-blend polymer solar cells enabled by a nonfullerene acceptor and two polymer donors with a broad composition tolerance. Adv. Mater. 29, 1704271 (2017).
Fei, Z. et al. An alkylated indacenodithieno[3,2-b]thiophene-based nonfullerene acceptor with high crystallinity exhibiting single junction solar cell efficiencies greater than 13% with low voltage losses. Adv. Mater. 30, 1705209 (2018).
Che, X., Li, Y., Qu, Y. & Forrest, S. R. High fabrication yield organic tandem photovoltaics combining vacuum- and solution-processed subcells with 15% efficiency. Nat. Energy 3, 422–427 (2018).
Huo, L. J. et al. Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv. Mater. 27, 2938 (2015).
Bin, H. et al. Non-fullerene polymer solar cells based on alkylthio and fluorine substituted 2D-conjugated polymers reach 9.5% efficiency. J. Am. Chem. Soc. 138, 4657–4664 (2016).
Fan, B. et al. High-performance nonfullerene polymer solar cells based on imide-functionalized wide-bandgap polymers. Adv. Mater. 29, 1606396 (2017).
Gao, H. et al. A new nonfullerene acceptor with near infrared absorption for high performance ternary-blend organic solar cells with efficiency over 13%. Adv. Sci. 5, 1800307 (2018).
Xiao, Z. et al. 26 mA cm−2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci. Bull. 62, 1494–1496 (2017).
Jia, B. et al. Breaking 10% efficiency in semitransparent solar cells with fused-undecacyclic electron acceptor. Chem. Mater. 30, 239–245 (2018).
Xie, S. et al. Effects of nonradiative losses at charge transfer states and energetic disorder on the open-circuit voltage in nonfullerene organic solar cells. Adv. Funct. Mater. 28, 1705659 (2018).
Li, N. et al. Abnormal strong burn-in degradation of highly efficient polymer solar cells caused by spinoidal donor-acceptor demixing. Nat. Commun. 8, 14541 (2017).
Zhang, C. et al. Overcoming the thermal instability of efficient polymer solar cells by employing novel fullerene-based acceptors. Adv. Energy Mater. 7, 1601204 (2017).
Zhao, W. et al. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 139, 7148–7151 (2017).
Dai, S. et al. Fused nonacyclic electron acceptors for efficient polymer solar cells. J. Am. Chem. Soc. 139, 1336–1343 (2017).
Lan, L. et al. High-performance polymer solar cells based on a wide-bandgap polymer containing pyrrolo[3,4-f]benzotriazole-5,7-dione with a power conversion efficiency of 8.63%. Adv. Sci. 3, 1600032 (2016).
Meager, I. et al. Photocurrent enhancement from diketopyrrolopyrrole polymer solar cells through alkyl-chain branching point manipulation. J. Am. Chem. Soc. 135, 11537–11540 (2013).
Lin, Y. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Ghosh, S., Li, X. Q., Stepanenko, V. & Wurthner, F. Control of H- and J-type π stacking by peripheral alkyl chains and self-sorting phenomena in perylene bisimide homo- and heteroaggregates. Chem. Eur. J. 14, 11343–11357 (2008).
Chan, J. M. W., Tischler, J. R., Kooi, S. E., Bulovic, V. & Swager, T. M. Synthesis of J-aggregating dibenz[a,j]anthracene-based macrocycles. J. Am. Chem. Soc. 131, 5659–5666 (2009).
Würthner, F., Kaiser, T. E. & Saha-Möller, C. R. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 50, 3376–3410 (2011).
Wu, Z. et al. n-Type water/alcohol-soluble naphthalene diimide-based conjugated polymers for high-performance polymer solar cells. J. Am. Chem. Soc. 138, 2004–2013 (2016).
Cheyns, D., Poortmans, J. & Heremans, P. Analytical model for the open-circuit voltage and its associated resistance in organic planar heterojunction solar cells. Phys. Rev. B 77, 165332 (2008).
Gasparini, N. et al. Designing ternary blend bulk heterojunction solar cells with reduced carrier recombination and a fill factor of 77%. Nat. Energy 1, 16118 (2016).
Tang, Z., Tress, W. & Inganäs, O. Light trapping in thin film organic solar cells. Mater. Today 17, 389–396 (2014).
Raut, H. K., Ganesh, V. A., Nair, A. S. & Ramakrishna, S. Anti-reflective coatings: a critical, in-depth review. Energy Environ. Sci. 4, 3779 (2011).
Nikolis, V. C. et al. Reducing voltage losses in cascade organic solar cells while maintaining high external quantum efficiencies. Adv. Energy Mater. 7, 1700855 (2017).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
Rau, U. Reciprocity relation between photovoltaic quantum efficiency and electroluminescent emission of solar cells. Phys. Rev. B 76, 085303 (2007).
Gao, F., Tress, W., Wang, J. & Inganas, O. Temperature dependence of charge carrier generation in organic photovoltaics. Phys. Rev. Lett. 114, 128701 (2015).
Zhao, W. et al. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 28, 4734–4739 (2016).
Baran, D. et al. Reduced voltage losses yield 10% efficient fullerene free organic solar cells with >1 V open circuit voltages. Energy Environ. Sci 9, 3783–3793 (2016).
Liu, X. et al. Efficient organic solar cells with extremely high open-circuit voltages and low voltage losses by suppressing nonradiative recombination losses. Adv. Energy Mater. 8, 1801699 (2018).
Vandewal, K. Interfacial charge transfer states in condensed phase systems. Annu. Rev. Phys. Chem. 67, 113–133 (2016).
Baran, D. et al. Role of polymer fractionation in energetic losses and charge carrier lifetimes of polymer: Fullerene solar cells. J. Phys. Chem. C 119, 19668–19673 (2015).
Acknowledgements
This work was financially supported by the Ministry of Science and Technology (no. 2014CB643501) and the National Natural Science Foundation of China (nos 91633301, 51521002, 51673069, 21520102006 and 21822505). N.L. gratefully acknowledges financial support from the DFG research grant BR 4031/13-1, the ETI funding at FAU Erlangen-Nürnberg, and the Bavarian Ministry of Economic Affairs and Media, Energy and Technology by funding the HI-ERN (IEK11) of FZ Jülich. C.J.B. gratefully acknowledges financial support through the ‘Aufbruch Bayern’ initiative of the state of Bavaria (EnCN and ‘Solar Factory of the Future’), the Bavarian Initiative ‘Solar Technologies go Hybrid’ (SolTech), the SFB 953 (DFG) and the Cluster of Excellence ‘Engineering of Advanced Materials’ (EAM) at FAU Erlangen-Nürnberg. RSoXS was performed at beamline 11.0.1.2 and GIWAXS was performed at beamline 7.3.3 at the Advanced Light Source of Lawrence Berkeley National Laboratory (LBNL), which was supported by the DOE, Office of Science and Office of Basic Energy Sciences. We acknowledge the support for film sample preparation at the Molecular Foundry, LBNL. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
B.F., L.Y., N.L. and F.H. conceived the ideas and coordinated the work. B.F. and L.Y. designed the donor polymers. B.F. synthesized the polymers, conducted the ultraviolet–visible and cyclic voltammetric measurements, performed the device fabrication and characterization, and analysed the data. X.D. and X.T. conducted the FTPS and EL measurements. X.D. analysed the FTPS and EL data. X.T. performed the temperature-dependent J–V characterization. R.X. synthesized the acceptor molecules. F.L., W.Z., J.X. and W.M. conducted the GIWAXS measurements and analysed the data. F.L. and W.Z. performed the RSoXS measurements and analysed the data. K.A. performed the light-intensity-dependent J–V characterization. N.L. assisted with the large-area device fabrication and evaluation. B.F., L.Y., N.L., C.J.B., F.H. and Y.C. contributed to manuscript preparation. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures 1–18, Supplementary tables 1–10, Supplementary references
Rights and permissions
About this article
Cite this article
Fan, B., Du, X., Liu, F. et al. Fine-tuning of the chemical structure of photoactive materials for highly efficient organic photovoltaics. Nat Energy 3, 1051–1058 (2018). https://doi.org/10.1038/s41560-018-0263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0263-4
This article is cited by
-
Mastering morphology of non-fullerene acceptors towards long-term stable organic solar cells
Nature Communications (2023)
-
A materials physics perspective on structure–processing–function relations in blends of organic semiconductors
Nature Reviews Materials (2023)
-
Enhanced photovoltaic performance of PM6/Y6-based organic solar cells by a wide-bandgap small molecule acceptor
Journal of Nanoparticle Research (2023)
-
Large-area Flexible Organic Solar Cells: Printing Technologies and Modular Design
Chinese Journal of Polymer Science (2022)
-
High-performance nonfused ring electron acceptor with a steric hindrance induced planar molecular backbone
Science China Chemistry (2022)