Abstract
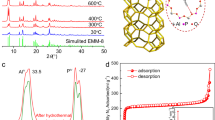
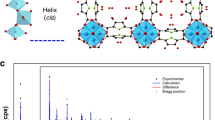
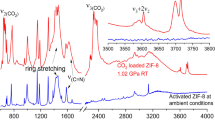
The discovery of more-efficient and stable water adsorbents for adsorption-driven chillers for cooling applications remains a challenge due to the low working capacity of water sorption, high regeneration temperature, low energy efficiency under given operating conditions and the toxicity risk of harmful working fluids for the state-of-the-art sorbents. Here we report the water-sorption properties of a porous zirconium carboxylate metal–organic framework, MIP-200, which features S-shaped sorption isotherms, a high water uptake of 0.39 g g−1 below P/P0 = 0.25, facile regeneration and stable cycling, and most importantly a notably high coefficient of performance of 0.78 for refrigeration at a low driving temperature (below 70 °C). A joint computational–experimental approach supports that MIP-200 may be a practical alternative to the current commercially available adsorbents for refrigeration when its water adsorption performance is combined with advantages such as the exceptional chemical and mechanical stability and the scalable synthesis that involves simple, cheap and green chemicals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The X-ray crystallographic data for MIP-200 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC 1834834. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk. Further data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Isaac, M. & van Vuuren, D. P. Modeling global residential sector energy demand for heating and air conditioning in the context of climate change. Energy Policy 37, 507–521 (2009).
Rogelj, J. et al. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 534, 631–639 (2016).
Ghafoor, A. & Munir, A. Worldwide overview of solar thermal cooling technologies. Renew. Sust. Energ. Rev. 43, 763–774 (2015).
de Lange, M. F. et al. Adsorption-driven heat pumps: the potential of metal–organic frameworks. Chem. Rev. 115, 12205–12250 (2015).
Enteria, N., Yoshino, H. & Mochida, A. Review of the advances in open-cycle absorption air-conditioning systems. Renew. Sust. Energ. Rev. 28, 265–289 (2013).
Khadiran, T., Hussein, M. Z., Zainal, Z. & Rusli, R. Advanced energy storage materials for building applications and their thermal performance characterization: a review. Renew. Sust. Energ. Rev. 57, 916–928 (2016).
Lizana, J., Chacartegui, R., Barrios-Padura, A. & Valverde, J. M. Advances in thermal energy storage materials and their applications towards zero energy buildings: a critical review. Appl. Energy 203, 219–239 (2017).
Sapienza, A. et al. Adsorption chilling driven by low temperature heat: new adsorbent and cycle optimization. Appl. Therm. Eng. 32, 141–146 (2012).
Aristov, Y. Concept of adsorbent optimal for adsorptive cooling/heating. Appl. Therm. Eng. 72, 166–175 (2014).
Wu, W. et al. Comparisons of different working pairs and cycles on the performance of absorption heat pump for heating and domestic hot water in cold regions. Appl. Therm. Eng. 48, 349–358 (2012).
de Lange, M. F. et al. Metal–organic frameworks in adsorption-driven heat pumps: the potential of alcohols as working fluids. Langmuir 31, 12783–12796 (2015).
Krajnc, A. et al. Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Adv. Energy Mater. 7, 8 (2017).
Freni, A. et al. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 82, 1–7 (2015).
Zhou, H. C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Maurin, G., Serre, C., Cooper, A. & Ferey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 46, 3104–3107 (2017).
Cadiau, A. et al. Design of hydrophilic metal organic framework water adsorbents for heat reallocation. Adv. Mater. 27, 4775–4780 (2015).
Seo, Y. K. et al. Energy-efficient dehumidification over hierarchically porous metal–organic frameworks as advanced water adsorbents. Adv. Mater. 24, 806–810 (2012).
Rieth, A. J., Yang, S., Wang, E. N. & Dinca, M. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit. ACS Central Sci. 3, 668–672 (2017).
AbdulHalim, R. G. et al. A fine-tuned metal–organic framework for autonomous indoor moisture control. J. Am. Chem. Soc. 139, 10715–10722 (2017).
Sohail, M. et al. Synthesis of highly crystalline NH2-MIL-125 (Ti) with S-shaped water isotherms for adsorption heat transformation. Cryst. Growth Des. 17, 1208–1213 (2017).
Permyakova, A. et al. Synthesis optimization, shaping, and heat reallocation evaluation of the hydrophilic metal–organic framework MIL-160(Al). ChemSusChem 10, 1419–1426 (2017).
Kummer, H. et al. A functional full-scale heat exchanger coated with aluminum fumarate metal–organic framework for adsorption heat transformation. Ind. Eng. Chem. Res. 56, 8393–8398 (2017).
Lenzen, D. et al. Scalable green synthesis and full-scale test of the metal–organic framework CAU-10-H for use in adsorption-driven chillers. Adv. Mater. 30, 1705869 (2018).
Furukawa, H. et al. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014).
Gordeeva, L. G., Solovyeva, M. V. & Aristov, Y. I. NH2-MIL-125 as a promising material for adsorptive heat transformation and storage. Energy 100, 18–24 (2016).
Srikhirin, P., Aphornratana, S. & Chungpaibulpatana, S. A review of absorption refrigeration technologies. Renew. Sust. Energ. Rev. 5, 343–372 (2001).
Lee, U. H., Valekar, A. H., Hwang, Y. K. & Chang, J.-S. in The Chemistry of Metal–Organic Frameworks: Synthesis, Characterization, and Applications (ed. Kaskel, S.) Ch. 18 (Wiley-VCH, Weinheim, 2016).
Bai, Y. et al. Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 45, 2327–2367 (2016).
Prat, D. et al. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 18, 288–296 (2016).
Duran, D. et al. PROXIMA 2A—a new fully tunable micro-focus beamline for macromolecular crystallography. J. Phys. Conf. Ser. 425, 012005 (2013).
Aristov, Y. I. Challenging offers of material science for adsorption heat transformation: a review. Appl. Therm. Eng. 50, 1610–1618 (2013).
Mileo, P. G. M. et al. Highly efficient proton conduction in a three-dimensional titanium hydrogen phosphate. Chem. Mat. 29, 7263–7271 (2017).
Borges, D. D. et al. Computational exploration of the water concentration dependence of the proton transport in the porous UiO-66(Zr)–(CO2H)2 metal–organic framework. Chem. Mat 29, 1569–1576 (2017).
Borges, D. D. et al. Proton transport in a highly conductive porous zirconium-based metal–organic framework: molecular insight. Angew. Chem. Int. Ed. 55, 3919–3924 (2016).
Chorowski, M. & Pyrka, P. Modelling and experimental investigation of an adsorption chiller using low-temperature heat from cogeneration. Energy 92, 221–229 (2015).
Elmegaard, B., Ommen, T. S., Markussen, M. & Iversen, J. Integration of space heating and hot water supply in low temperature district heating. Energy Build. 124, 255–264 (2016).
Krause, S. et al. A stimuli-responsive zirconium metal–organic framework based on supermolecular design. Angew. Chem. Int. Ed. 56, 10676–10680 (2017).
Jiang, J. et al. Superacidity in sulfated metal–organic framework-808. J. Am. Chem. Soc. 136, 12844–12847 (2014).
Mouchaham, G. et al. A robust infinite zirconium phenolate building unit to enhance the chemical stability of Zr MOFs. Angew. Chem. Int. Ed. 54, 13297–13301 (2015).
Guillerm, V. et al. A series of isoreticular, highly stable, porous zirconium oxide based metal–organic frameworks. Angew. Chem. Int. Ed. 51, 9267–9271 (2012).
Yuan, S. et al. Stable metal-organic frameworks: design, synthesis, and applications. Adv. Mater. 30, 1704303 (2018).
Howarth, A. J. et al. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 1, 15 (2016).
Bennett, T. D. & Cheetham, A. K. Amorphous metal–organic frameworks. Accounts Chem. Res. 47, 1555–1562 (2014).
Wang, J., Evans, A. G., Dharmasena, K. & Wadley, H. N. G. On the performance of truss panels with Kagome cores. Int. J. Solids Struct. 40, 6981–6988 (2003).
Zhang, Y. H., Qu, X. M. & Fang, D. N. Mechanical properties of two novel planar lattice structures. Int. J. Solids Struct. 45, 3751–3768 (2008).
Gomez-Gualdron, D. A. et al. Computational design of metal–organic frameworks based on stable zirconium building units for storage and delivery of methane. Chem. Mat. 26, 5632–5639 (2014).
Liu, T. F. et al. Topology-guided design and syntheses of highly stable mesoporous porphyrinic zirconium metal–organic frameworks with high surface area. J. Am. Chem. Soc. 137, 413–419 (2015).
VandeVondele, J. et al. QUICKSTEP: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. CP2K: atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 4, 15–25 (2014).
The CP2K Developers Group (accessed 1 August 2018); http://www.cp2k.org
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 25, 1463–1473 (2004).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 114, 145–152 (2005).
Hartwigsen, C., Goedecker, S. & Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B 58, 3641–3662 (1998).
Yang, Q. Y. & Zhong, C. L. Understanding hydrogen adsorption in metal–organic frameworks with open metal sites: a computational study. J. Phys. Chem. B 110, 655–658 (2006).
Rappe, A. K. et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992).
Mayo, S. L., Olafson, B. D. & Goddard, W. A. DREIDING: a generic force field for molecular simulations. J. Phys. Chem. 94, 8897–8909 (1990).
Abascal, J. L. & Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 123, 234505 (2005).
Vlugt, T. J. et al. Computing the heat of adsorption using molecular simulations: the effect of strong coulombic interactions. J. Chem. Theory Comput. 4, 1107–1118 (2008).
Acknowledgements
S.W., G.M. and C.S. acknowledge the financial support of the European Community within the Seventh Framework Programme (FP7) under grant agreement no. 608490 (Project M4CO2). C.M.-C. and G.M. are grateful for financial support from Institut Universitaire de France (IUF). The Korean authors are grateful to the Global Frontier Center for Hybrid Interface Materials of Korea (GFHIM) (grant no. NRF-2013M3A6B1078879) and the National Research Council of Science & Technology (NST) of Korea (the R&D Convergence Program, Center for Convergent Chemical Process (CCP), CRC-14-1-KRICT) for financial support. E. Gkaniatsou and L. Zhou from the Institute of Porous Materials from Paris, and A. Orsi and P. Wright from the University of St Andrews are acknowledged for providing the MOF samples for the chemical stability tests under NH4OH vapour. J. W. Yoon, Y. K. Hwang, U-H. Lee and H. Wang are also acknowledged for their fruitful comments on adsorption and characterization experiments.
Author information
Authors and Affiliations
Contributions
S.W. contributed to the synthesis and general characterization of MIP-200 and contributed to the writing of the manuscript. M.W. performed the computational assisted structure determination of MIL-200, the calculation of the electronic energy for a series of Zr-based MOFs, the simulation of the water adsorption isotherms and the analysis of the mechanism in play, and he equally contributed to the writing of the manuscript. M.M. contributed to the characterization data collection and writing of the manuscript. A.T., J.M. and W.S. collected the synchrotron diffraction data on the single crystal and solved the crystal structure. C.M.-C. conducted the solid-state NMR characterizations. J.S.L. and K.H.C. collected water-sorption data. J.P. calculated the thermodynamic efficiency of MIP-200 for water sorption. J.-S.C. designed the study on water sorption, analysed data and led the writing of the manuscript. G.M. supervised the modelling part of this study and led the writing of the manuscript. C.S. coordinated the study and led the writing of the manuscript, and also closely supervised the synthesis and characterization part of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures 1–40, Supplementary tables 1–5, Supplementary notes 1–3, Supplementary methods, Supplementary references
Rights and permissions
About this article
Cite this article
Wang, S., Lee, J.S., Wahiduzzaman, M. et al. A robust large-pore zirconium carboxylate metal–organic framework for energy-efficient water-sorption-driven refrigeration. Nat Energy 3, 985–993 (2018). https://doi.org/10.1038/s41560-018-0261-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0261-6
This article is cited by
-
Progress toward the computational discovery of new metal–organic framework adsorbents for energy applications
Nature Energy (2024)
-
Sustainable moisture energy
Nature Reviews Materials (2024)
-
A robust ultra-microporous cationic aluminum-based metal-organic framework with a flexible tetra-carboxylate linker
Communications Chemistry (2023)
-
A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation
Nature Communications (2023)
-
Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks
Nature Catalysis (2023)