Abstract
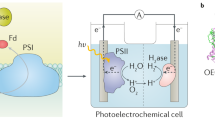
Natural photosynthesis stores sunlight in chemical energy carriers, but it has not evolved for the efficient synthesis of fuels, such as H2. Semi-artificial photosynthesis combines the strengths of natural photosynthesis with synthetic chemistry and materials science to develop model systems that overcome nature’s limitations, such as low-yielding metabolic pathways and non-complementary light absorption by photosystems I and II. Here, we report a bias-free semi-artificial tandem platform that wires photosystem II to hydrogenase for overall water splitting. This photoelectrochemical cell integrated the red and blue light-absorber photosystem II with a green light-absorbing diketopyrrolopyrrole dye-sensitized TiO2 photoanode, and so enabled complementary panchromatic solar light absorption. Effective electronic communication at the enzyme–material interface was engineered using an osmium-complex-modified redox polymer on a hierarchically structured TiO2. This system provides a design protocol for bias-free semi-artificial Z schemes in vitro and provides an extended toolbox of biotic and abiotic components to re-engineer photosynthetic pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sakimoto, K. K., Wong, A. B. & Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351, 74–77 (2016).
Yehezkeli, O. et al. Integrated photosystem II-based photo-bioelectrochemical cells. Nat. Commun. 3, 742 (2012).
Mersch, D. et al. Wiring of photosystem II to hydrogenase for photoelectrochemical water-splitting. J. Am. Chem. Soc. 137, 8541–8549 (2015).
Sokol, K. P. et al. Rational wiring of photosystem II to hierarchical indium tin oxide electrodes using redox polymers. Energy Environ. Sci. 9, 3698–3709 (2016).
Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl Acad. Sci. USA 112, 8529–8536 (2015).
Tachibana, Y., Vayssieres, L. & Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photon. 6, 511–518 (2012).
Woolerton, T. W., Sheard, S., Chaudhary, Y. S. & Armstrong, F. A. Enzymes and bio-inspired electrocatalysts in solar fuel devices. Energy Environ. Sci. 5, 7470–7490 (2012).
Léger, C. & Bertrand, P. Direct electrochemistry of redox enzymes as a tool for mechanistic studies. Chem. Rev. 108, 2379–2438 (2008).
Kato, M., Zhang, J. Z., Paul, N. & Reisner, E. Protein film photoelectrochemistry of the water oxidation enzyme photosystem II. Chem. Soc. Rev. 43, 6485–6497 (2014).
Bard, A. J. & Fox, M. A. Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 28, 141–145 (1995).
Govindjee, Shevela, D. & Björn, L. O. Evolution of the Z-scheme of photosynthesis: a perspective. Photosynth. Res. 133, 5–15 (2017).
Barber, J. & Tran, P. D. From natural to artificial photosynthesis. J. R. Soc. Interface 10, 20120984 (2013).
Khetkorn, W. et al. Microalgal hydrogen production—a review. Bioresource Technol. 243, 1194–1206 (2017).
Kruse, O., Rupprecht, J., Mussgnug, J. H., Dismukes, G. C. & Hankamer, B. Photosynthesis: a blueprint for solar energy capture and biohydrogen production technologies. Photochem. Photobiol. Sci. 4, 957–969 (2005).
Michel, H. The nonsense of biofuels. Angew. Chem. Int. Ed. 51, 2516–2518 (2012).
Esper, B., Badura, A. & Rögner, M. Photosynthesis as a power supply for (bio-)hydrogen production. Trends Plant Sci. 11, 543–549 (2006).
Hu, S., Xiang, C., Haussener, S., Berger, A. D. & Lewis, N. S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 6, 2984–2993 (2013).
Kothe, T. et al. Combination of a photosystem I-based photocathode and a photosystem II-based photoanode to a Z-scheme mimic for biophotovoltaic applications. Angew. Chem. Int. Ed. 52, 14233–14236 (2013).
Hartmann, V. et al. Redox hydrogels with adjusted redox potential for improved efficiency in Z-scheme inspired biophotovoltaic cells. Phys. Chem. Chem. Phys. 16, 11936–11941 (2014).
Kim, Y. et al. Hybrid Z-scheme using photosystem I and BiVO4 for hydrogen production. Adv. Funct. Mater. 25, 2369–2377 (2015).
Rao, K. K. et al. Photoelectrochemical responses of photosystem II particles immobilized on dye-derivatized TiO2 films. J. Photochem. Photobiol. B 5, 379–389 (1990).
Wang, W. et al. Spatially separated photosystem II and a silicon photoelectrochemical cell for overall water splitting: a natural–artificial photosynthetic hybrid. Angew. Chem. Int. Ed. 55, 9229–9233 (2016).
Pinhassi, R. I. et al. Hybrid bio-photo-electro-chemical cells for solar water splitting. Nat. Commun. 7, 12552 (2016).
O’Regan, B. & Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitised colloidal TiO2 films. Nature 353, 737–740 (1991).
Xu, P., McCool, N. S. & Mallouk, T. E. Water splitting dye-sensitized solar cells. Nano Today 14, 42–58 (2017).
Warnan, J. et al. A compact diketopyrrolopyrrole dye as efficient sensitizer in titanium dioxide dye-sensitized solar cells. J. Photochem. Photobiol. A 226, 9–15 (2011).
Warnan, J. et al. Solar H2 evolution in water with modified diketopyrrolopyrrole dyes immobilised on molecular Co and Ni catalyst–TiO2 hybrids. Chem. Sci. 8, 3070–3079 (2017).
Muresan, N. M., Willkomm, J., Mersch, D., Vaynzof, Y. & Reisner, E. Immobilization of a molecular cobaloxime catalyst for hydrogen evolution on a mesoporous metal oxide electrode. Angew. Chem. Int. Ed. 51, 12749–12753 (2012).
Lakadamyali, F., Reynal, A., Kato, M., Durrant, J. R. & Reisner, E. Electron transfer in dye-sensitised semiconductors modified with molecular cobalt catalysts: photoreduction of aqueous protons. Chem. Eur. J. 18, 15464–15475 (2012).
Knauf, R. R., Brennaman, M. K., Alibabaei, L., Norris, M. R. & Dempsey, J. L. Revealing the relationship between semiconductor electronic structure and electron transfer dynamics at metal oxide−chromophore interfaces. J. Phys. Chem. C 117, 25259–25268 (2013).
Li, F. et al. Immobilizing Ru(bda) catalyst on a photoanode via electrochemical polymerization for light-driven water splitting. ACS Catal. 5, 3786–3790 (2015).
Willkomm, J. et al. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 45, 9–23 (2016).
Umena, Y., Kawakami, K., Shen, J.-R. & Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011).
Rapatskiy, L. et al. Detection of the water-binding sites of the oxygen-evolving complex of photosystem II using W-band 17O electron−electron double resonance-detected NMR spectroscopy. J. Am. Chem. Soc. 134, 16619–16634 (2012).
Kuhl, H. et al. Towards structural determination of the water-splitting enzyme: purification, crystallization, and preliminary crystallographic studies of photosystem II from a thermophilic cyanobacterium. J. Biol. Chem. 275, 20652–20659 (2000).
Kern, J. et al. Purification, characterisation and crystallisation of photosystem II from Thermosynechococcus elongatus cultivated in a new type of photobioreactor. Biochim. Biophys. Acta Bioenerg. 1706, 147–157 (2005).
Badura, A. et al. Photo-induced electron transfer between photosystem II via cross-linked redox hydrogels. Electroanalysis 20, 1043–1047 (2008).
Senge, M. O., Ryan, A. A., Letchford, K. A., MacGowan, S. A. & Mielke, T. Chlorophylls, symmetry, chirality, and photosynthesis. Symmetry 6, 781–843 (2014).
Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 80–83 (2011).
Zhang, J. Z. et al. Competing charge transfer pathways at the photosystem II–electrode interface. Nat. Chem. Biol. 12, 1046–1052 (2016).
Razeghifard, R. & Wydrzynski, T. J. Artificial Photosynthesis: From Basic Biology to Industrial Application (Wiley, Hoboken, 2007).
Reisner, E., Powell, D. J., Cavazza, C., Fontecilla-Camps, J. C. & Armstrong, F. A. Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles. J. Am. Chem. Soc. 131, 18457–18466 (2009).
Wombwell, C., Caputo, C. A. & Reisner, E. [NiFeSe]-hydrogenase chemistry. Acc. Chem. Res. 48, 2858–2865 (2015).
Hambourger, M. et al. [FeFe]-hydrogenase-catalyzed H2 production in a photoelectrochemical biofuel cell. J. Am. Chem. Soc. 130, 2015–2022 (2008).
Coridan, R. H. et al. Methods for comparing the performance of energy-conversion systems for use in solar fuels and solar electricity generation. Energy Environ. Sci. 8, 2886–2901 (2015).
Cai, P. et al. Co-assembly of photosystem II/reduced graphene oxide multilayered biohybrid films for enhanced photocurrent. Nanoscale 7, 10908–10911 (2015).
Dotan, H., Mathews, N., Hisatomi, T., Grätzel, M. & Rothschild, A. On the solar to hydrogen conversion efficiency of photoelectrodes for water splitting. J. Phys. Chem. Lett. 5, 3330–3334 (2014).
Hatchikian, E. C., Bruschi, M. & Le Gall, J. Characterisation of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 82, 451–461 (1978).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 975, 384–394 (1989).
Acknowledgements
This work was supported by an ERC Consolidator Grant MatEnSAP (682833), the UK Engineering and Physical Sciences Research Council (EP/L015978/1 and EP/G037221/1, nanoDTC and a DTA studentship), the Christian Doppler Research Association, the OMV Group and a Royal Society Newton International Fellowship (NF160054), the Cluster of Excellence RESOLV (EXC 1069) funded by the Deutsche Forschungsgemeinschaft and the European Union’s Horizon 2020 MSCA ITN-EJD 764920 PHOTOBIOCAT. The HAADF–STEM was carried out at the National Center of Electron Microscopy (NCEM), which is supported by the Office of Science, Office of Basic Energy Sciences of the US Department of Energy under Contract no. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences of the US Department of Energy under Contract no. DE-AC02-05CH11231. We thank J. Fontecilla-Camps and C. Cavazza for providing the H2ase enzyme, V. Hartmann for his contribution to the PSII preparation and N. Plumeré, C. Creissen, S. Kalathil and N. Heidary for valuable discussions.
Author information
Authors and Affiliations
Contributions
K.P.S., W.E.R., J.Z.Z. and E.R. conceived the research. K.P.S. prepared and characterized the electrodes and performed the electrochemical experiments. W.E.R. helped with the experiment design and supported the electrochemical experiments. J.W. synthesized the dpp dye. N.K. carried out the HAADF–STEM and RRDE measurements. M.M.N. provided the PSII samples. A.R. synthesized the POs polymer. K.P.S., W.E.R., N.K., J.Z.Z. and E.R. analysed the data. All the authors contributed to the creation of the manuscript. E.R. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Supplementary Figures 1–19
Rights and permissions
About this article
Cite this article
Sokol, K.P., Robinson, W.E., Warnan, J. et al. Bias-free photoelectrochemical water splitting with photosystem II on a dye-sensitized photoanode wired to hydrogenase. Nat Energy 3, 944–951 (2018). https://doi.org/10.1038/s41560-018-0232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0232-y
This article is cited by
-
Thermophilic cyanobacteria—exciting, yet challenging biotechnological chassis
Applied Microbiology and Biotechnology (2024)
-
A functional hydrogenase mimic that catalyzes robust H2 evolution spontaneously in aqueous environment
Nano Research (2024)
-
Bias-free driven ion assisted photoelectrochemical system for sustainable wastewater treatment
Nature Communications (2023)
-
Adaptive insertion of a hydrophobic anchor into a poly(ethylene glycol) host for programmable surface functionalization
Nature Chemistry (2023)
-
Rewiring photosynthetic electron transport chains for solar energy conversion
Nature Reviews Bioengineering (2023)