Abstract
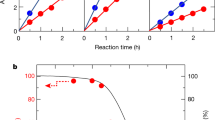
Full water splitting into hydrogen and oxygen on semiconductor nanocrystals is a challenging task; overpotentials must be overcome for both half-reactions and different catalytic sites are needed to facilitate them. Additionally, efficient charge separation and prevention of back reactions are necessary. Here, we report simultaneous H2 and O2 evolution by CdS nanorods decorated with nanoparticulate reduction and molecular oxidation co-catalysts. The process proceeds entirely without sacrificial agents and relies on the nanorod morphology of CdS to spatially separate the reduction and oxidation sites. Hydrogen is generated on Pt nanoparticles grown at the nanorod tips, while Ru(tpy)(bpy)Cl2-based oxidation catalysts are anchored through dithiocarbamate bonds onto the sides of the nanorod. O2 generation from water was verified by 18O isotope labelling experiments, and time-resolved spectroscopic results confirmed efficient charge separation and ultrafast electron and hole transfer to the reaction sites. The system demonstrates that combining nanoparticulate and molecular catalysts on anisotropic nanocrystals provides an effective pathway for visible-light-driven photocatalytic water splitting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, N. S. Toward cost-effective solar energy use. Science 315, 798–801 (2007).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Frischmann, P. D., Mahata, K. & Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 42, 1847–1870 (2013).
Tinker, L. L., McDaniel, N. D. & Bernhard, S. Progress towards solar-powered homogeneous water photolysis. J. Mater. Chem. 19, 3328–3337 (2009).
Tran, P. D., Artero, V. & Fontecave, M. Water electrolysis and photoelectrolysis on electrodes engineered using biological and bio-inspired molecular systems. Energy Environ. Sci. 3, 727–747 (2010).
Osterloh, F. E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 42, 2294–2320 (2013).
Simon, T. et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 13, 1013–1018 (2014).
Duan, L., Tong, L., Xu, Y. & Sun, L. Visible light-driven water oxidation-from molecular catalysts to photoelectrochemical cells. Energy Environ. Sci. 4, 3296–3313 (2011).
Gust, D., Moore, T. A. & Moore, A. L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 42, 1890–1898 (2009).
Hetterscheid, D. G. H. & Reek, J. N. H. Mononuclear water oxidation catalysts. Angew. Chem. Int. Ed. 51, 9740–9747 (2012).
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011).
Gao, Y. et al. Visible light driven water splitting in a molecular device with unprecedentedly high photocurrent density. J. Am. Chem. Soc. 135, 4219–4222 (2013).
Symes, M. D. & Cronin, L. Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 5, 403–409 (2013).
Wang, X. et al. Photocatalytic overall water splitting promoted by an α–β phase junction on Ga2O3. Angew. Chem. Int. Ed. 51, 13089–13092 (2012).
Maeda, K. & Domen, K. Photocatalytic water splitting: recent progress and future challenges. J. Phys. Chem. Lett. 1, 2655–2661 (2010).
Han, Z., Qiu, F., Eisenberg, R., Holland, P. L. & Krauss, T. D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 338, 1321–1324 (2012).
Ben-Shahar, Y. et al. Optimal metal domain size for photocatalysis with hybrid semiconductor–metal nanorods. Nat. Commun. 7, 10413 (2016).
Wu, K. & Lian, T. Quantum confined colloidal nanorod heterostructures for solar-to-fuel conversion. Chem. Soc. Rev. 45, 3781–3810 (2016).
Stolarczyk, J. K., Bhattacharyya, S., Polavarapu, L. & Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 8, 3602–3635 (2018).
Simon, T., Carlson, M. T., Stolarczyk, J. K. & Feldmann, J. Electron transfer rate vs recombination losses in photocatalytic H2 generation on Pt-decorated CdS nanorods. ACS Energy Lett. 1, 1137–1142 (2016).
Kalisman, P., Nakibli, Y. & Amirav, L. Perfect photon-to-hydrogen conversion efficiency. Nano Lett. 16, 1776–1781 (2016).
Wu, K. et al. Hole removal rate limits photodriven H2 generation efficiency in CdS-Pt and CdSe/CdS-Pt semiconductor nanorod–metal tip heterostructures. J. Am. Chem. Soc. 136, 7708–7716 (2014).
Chen, S. & Wang, L.-W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 24, 3659–3666 (2012).
Berr, M. J. et al. Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation. Appl. Phys. Lett. 100, 223903 (2012).
Ding, T. X., Olshansky, J. H., Leone, S. R. & Alivisatos, A. P. Efficiency of hole transfer from photoexcited quantum dots to covalently linked molecular species. J. Am. Chem. Soc. 137, 2021–2029 (2015).
Kalyanasundaram, K., Borgarello, E., Duonghong, D. & Grätzel, M. Cleavage of water by visible-light irradiation of colloidal CdS solutions; inhibition of photocorrosion by RuO2. Angew. Chem. Int. Ed. 20, 987–988 (1981).
Kalisman, P., Kauffmann, Y. & Amirav, L. Photochemical oxidation on nanorod photocatalysts. J. Mater. Chem. A 3, 3261–3265 (2015).
Concepcion, J. J. et al. Making oxygen with ruthenium complexes. Acc. Chem. Res. 42, 1954–1965 (2009).
Tseng, H.-W., Wilker, M. B., Damrauer, N. H. & Dukovic, G. Charge transfer dynamics between photoexcited CdS nanorods and mononuclear Ru water-oxidation catalysts. J. Am. Chem. Soc. 135, 3383–3386 (2013).
Thackeray, J. W., Natan, M. J., Ng, P. & Wrighton, M. S. Interaction of diethyldithiocarbamate with n-type cadmium sulfide and cadmium selenide: efficient photoelectrochemical oxidation to the disulfide and flat-band potential of the semiconductor as a function of adsorbate concentration. J. Am. Chem. Soc. 108, 3570–3577 (1986).
La Croix, A. D. et al. Design of a hole trapping ligand. Nano Lett. 17, 909–914 (2017).
Saunders, A. E., Ghezelbash, A., Sood, P. & Korgel, B. A. Synthesis of high aspect ratio quantum-size CdS nanorods and their surface-dependent photoluminescence. Langmuir 24, 9043–9049 (2008).
Mokari, T., Rothenberg, E., Popov, I., Costi, R. & Banin, U. Selective growth of metal tips onto semiconductor quantum rods and tetrapods. Science 304, 1787–1790 (2004).
Klimov, V. I. Optical nonlinearities and ultrafast carrier dynamics in semiconductor nanocrystals. J. Phys. Chem. B 104, 6112–6123 (2000).
Wu, K., Zhu, H., Liu, Z., Rodríguez-Córdoba, W. & Lian, T. Ultrafast charge separation and long-lived charge separated state in photocatalytic CdS–Pt nanorod Hhterostructures. J. Am. Chem. Soc. 134, 10337–10340 (2012).
Utterback, J. K. et al. Observation of trapped-hole diffusion on the surfaces of CdS nanorods. Nat. Chem. 8, 1061–1066 (2016).
Lian, S., Weinberg, D. J., Harris, R. D., Kodaimati, M. S. & Weiss, E. A. Subpicosecond photoinduced hole transfer from a CdS quantum dot to a molecular acceptor bound through an exciton-delocalizing ligand. ACS Nano 10, 6372–6382 (2016).
Lee, J. R., Li, W., Cowan, A. J. & Jäckel, F. Hydrophilic, hole-delocalizing ligand shell to promote charge transfer from colloidal CdSe quantum dots in water. J. Phys. Chem. C 121, 15160–15168 (2017).
Habas, S. E., Yang, P. & Mokari, T. Selective growth of metal and binary metal tips on CdS nanorods. J. Am. Chem. Soc. 130, 3294–3295 (2008).
Yu, W. W., Qu, L., Guo, W. & Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 15, 2854–2860 (2003).
Acknowledgements
This work was supported by the Bavarian State Ministry of Science, Research, and Arts through the grant ‘Solar Technologies go Hybrid (SolTech)’ and the ERANETMED programme (project Hydrosol, grant no. ENERG-11-132). The authors thank C. Hohmann (Nanosystems Initiative Munich) for his support with graphics design. P.D.F. thanks the Alexander von Humboldt Foundation for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the experiments, interpretation of the results and discussion of the outline of the manuscript. C.M.W., P.D.F., M.S., B.J.B., R.W., P.L. and M.T.C. carried out the experiments. J.K.S. wrote the manuscript, with input and comments from the other authors. J.F., F.W. and J.K.S. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Notes 1–2, Supplementary Tables 1–2, Supplementary Figures 1–20, Supplementary References 1–2
Rights and permissions
About this article
Cite this article
Wolff, C.M., Frischmann, P.D., Schulze, M. et al. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods. Nat Energy 3, 862–869 (2018). https://doi.org/10.1038/s41560-018-0229-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0229-6
This article is cited by
-
Synergistic effect of homojunction and Ohmic junctions in CdS boosting spatial charge separation for U(VI) photoreduction
Nano Research (2024)
-
Overcoming small-bandgap charge recombination in visible and NIR-light-driven hydrogen evolution by engineering the polymer photocatalyst structure
Nature Communications (2024)
-
Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production
Nature Nanotechnology (2023)
-
In situ constructing atomic interface in ruthenium-based amorphous hybrid-structure towards solar hydrogen evolution
Nature Communications (2023)
-
Hydrovoltaic effect-enhanced photocatalysis by polyacrylic acid/cobaltous oxide–nitrogen doped carbon system for efficient photocatalytic water splitting
Nature Communications (2023)