Abstract
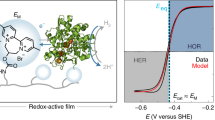
Enzymes are promising electrocatalysts for electron transfer (ET) in many biological processes. Strategies to enhance ET between enzymes and electroactive surfaces include orientation and immobilization of the enzymes and electron mediation. Here, we develop a strategy to couple orientation and electron mediation on electrodes based on carbon nanotubes. This is achieved by the synthesis of a redox mediator that contains an enzyme-orientation site (pyrene), an electron-carrier redox mediator (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)) and an electropolymerizable monomer (pyrrole). The coupling of an enzymatic orientation and a mediated ET in the same chemical structure (pyrrole–ABTS–pyrene (pyrr–ABTS–pyr)) provides a much-improved performance in the bioelectrocatalysis. We demonstrate two fuel cells for the synthesized redox mediator. In a proton-exchange membrane hydrogen/air fuel cell and in a membraneless fuel cell, the pyrr–ABTS–pyr biocathode provides a power density of 1.07 mW cm−2 and 7.9 mW cm−2, respectively. The principle of coupling an enzyme orientation and a redox mediator allows a great variety of mediators to be engineered and provides vast possibilities for the development of fuel cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Calabrese Barton, S., Gallaway, J. & Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 104, 4867–4886 (2004).
Cooney, M. J., Svoboda, V., Lau, C., Martin, G. & Minteer, S. D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 1, 320–337 (2008).
Rubin, E. M. Genomics of cellulosic biofuels. Nature 454, 841–845 (2008).
Elouarzaki, K. et al. Freestanding HRP–GOx redox buckypaper as an oxygen-reducing biocathode for biofuel cell applications. Energy Environ. Sci. 8, 2069–2074 (2015).
Yehezkeli, O. et al. Integrated photosystem II-based photo-bioelectrochemical cells. Nat. Commun. 3, 742 (2012).
Frew, J. E. & Hill, H. A. O. Direct and indirect electron transfer between electrodes and redox proteins. Eur. J. Biochem. 172, 261–269 (1988).
Fultz, M. L. & Durst, R. A. Mediator compounds for the electrochemical study of biological redox systems: a compilation. Anal. Chim. Acta 140, 1–18 (1982).
Sund, C. J., McMasters, S., Crittenden, S. R., Harrell, L. E. & Sumner, J. J. Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl. Microbiol. Biotechnol. 76, 561–568 (2007).
Belevich, I., Verkhovsky, M. I. & Wikström, M. Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature 440, 829–832 (2006).
Gust, D., Moore, T. A. & Moore, A. L. Molecular mimicry of photosynthetic energy and electron transfer. Acc. Chem. Res. 26, 198–205 (1993).
Moser, C. C., Keske, J. M., Warncke, K., Farid, R. S. & Dutton, P. L. Nature of biological electron transfer. Nature 355, 796–802 (1992).
Zebda, A. et al. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube–enzyme electrodes. Nat. Commun. 2, 370 (2011).
Agnes, C. et al. Supercapacitor/biofuel cell hybrids based on wired enzymes on carbon nanotube matrices: autonomous reloading after high power pulses in neutral buffered glucose solutions. Energy Environ. Sci. 7, 1884–1888 (2014).
Bourourou, M. et al. Freestanding redox buckypaper electrodes from multi-wall carbon nanotubes for bioelectrocatalytic oxygen reduction via mediated electron transfer. Chem. Sci. 5, 2885–2888 (2014).
Chaudhuri, S. K. & Lovley, D. R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotech. 21, 1229–1232 (2003).
Gao, F., Viry, L., Maugey, M., Poulin, P. & Mano, N. Engineering hybrid nanotube wires for high-power biofuel cells. Nat. Commun. 1, 2 (2010).
Shleev, S., Pita, M., Yaropolov, A. I., Ruzgas, T. & Gorton, L. Direct heterogeneous electron transfer reactions of Trametes hirsuta laccase at bare and thiol-modified gold electrodes. Electroanalysis 18, 1901–1908 (2006).
Solomon, E. I., Szilagyi, R. K., DeBeer George, S. & Basumallick, L. Electronic structures of metal sites in proteins and models: contributions to function in blue copper proteins. Chem. Rev. 104, 419–458 (2004).
Bertrand, T. et al. Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry 41, 7325–7333 (2002).
Piontek, K., Antorini, M. & Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 277, 37663–37669 (2002).
Enguita, F. J. et al. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J. Biol. Chem. 279, 23472–23476 (2004).
Blanford, C. F., Heath, R. S. & Armstrong, F. A. A stable electrode for high-potential, electrocatalytic O2 reduction based on rational attachment of a blue copper oxidase to a graphite surface. Chem. Commun. 0, 1710–1712 (2007).
Lalaoui, N., Elouarzaki, K., Goff, A. L., Holzinger, M. & Cosnier, S. Efficient direct oxygen reduction by laccases attached and oriented on pyrene-functionalized polypyrrole/carbon nanotube electrodes. Chem. Commun. 49, 9281–9283 (2013).
Bourourou, M. et al. Supramolecular immobilization of laccase on carbon nanotube electrodes functionalized with (methylpyrenylaminomethyl)anthraquinone for direct electron reduction of oxygen. Chem. Eur. J. 19, 9371–9375 (2013).
Mano, N. et al. Oxygen Is electroreduced to water on a 'wired' enzyme electrode at a lesser overpotential than on platinum. J. Am. Chem. Soc. 125, 15290–15291 (2003).
Soukharev, V., Mano, N. & Heller, A. A four-electron O2-electroreduction biocatalyst superior to platinum and a biofuel cell operating at 0.88 V. J. Am. Chem. Soc. 126, 8368–8369 (2004).
Cardoso, F. P. et al. Biocathodes for enzymatic biofuel cells using laccase and different redox mediators entrapped in polypyrrole matrix. J. Electrochem. Soc. 161, F445–F450 (2014).
Chen, T. et al. A miniature biofuel cell. J. Am. Chem. Soc. 123, 8630–8631 (2001).
Kiiskinen, L.-L., Viikari, L. & Kruus, K. Purification and characterisation of a novel laccase from the ascomycete Melanocarpus albomyces. Appl. Microbiol. Biotechnol. 59, 198–204 (2002).
Lee, J. Y., Shin, H. Y., Kang, S. W., Park, C. & Kim, S. W. Use of bioelectrode containing DNA-wrapped single-walled carbon nanotubes for enzyme-based biofuel cell. J. Power Sources 195, 750–755 (2010).
Lalaoui, N. et al. Hosting adamantane in the substrate pocket of laccase: direct bioelectrocatalytic reduction of O2 on functionalized carbon nanotubes. ACS Catal. 6, 4259–4264 (2016).
Krishnan, S. & Armstrong, F. A. Order-of-magnitude enhancement of an enzymatic hydrogen-air fuel cell based on pyrenyl carbon nanostructures. Chem. Sci. 3, 1015–1023 (2012).
Wang, Y., Esterle, T. F. & Armstrong, F. A. Electrocatalysis by H2–O2 membrane-free fuel cell enzymes in aqueous microenvironments confined by an ionic liquid. RSC Adv. 6, 44129–44134 (2016).
Xu, L. & Armstrong, F. A. Optimizing the power of enzyme-based membrane-less hydrogen fuel cells for hydrogen-rich H2–air mixtures. Energy Environ. Sci. 6, 2166–2171 (2013).
Ding, S.-N., Cosnier, S., Holzinger, M. & Wang, X. Electrochemical fabrication of novel fluorescent polymeric film: poly(pyrrole–pyrene). Electrochem. Commun. 10, 1423–1426 (2008).
Tsujimura, S., Fujita, M., Tatsumi, H., Kano, K. & Ikeda, T. Bioelectrocatalysis-based dihydrogen/dioxygen fuel cell operating at physiological pH. Phys. Chem. Chem. Phys. 3, 1331–1335 (2001).
Tarasevich, M. R., Bogdanovskaya, V. A., Zagudaeva, N. M. & Kapustin, A. V. Composite materials for direct bioelectrocatalysis of the hydrogen and oxygen reactions in biofuel cells. Russ. J. Electrochem. 38, 335–335 (2002).
Villalonga, R. et al. Supramolecular assembly of β-cyclodextrin-modified gold nanoparticles and Cu, Zn-superoxide dismutase on catalase. J. Mol. Catal. B 35, 79–85 (2005).
Acknowledgements
This project is funded by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Author information
Authors and Affiliations
Contributions
K.E., A.C.F. and J.-M.L. designed the chemical structures. K.E. developed the protocol for the organic synthesis. D.C. made the fuel cell reactor. K.E. and A.F. carried out the electrochemical characterization. J.-M.L. designed the electrode functionalized with polymers and Lac, and K.E. conducted all the experiments. J.-M.L. was responsible for the project management. K.E and J.-M.L. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7
Rights and permissions
About this article
Cite this article
Elouarzaki, K., Cheng, D., Fisher, A.C. et al. Coupling orientation and mediation strategies for efficient electron transfer in hybrid biofuel cells. Nat Energy 3, 574–581 (2018). https://doi.org/10.1038/s41560-018-0166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0166-4
This article is cited by
-
Electron highways
Nature Energy (2018)